Executive/Sr. Executive to Manager – Drug Regulatory Affairs at Gracure Pharmaceuticals Ltd

Are you looking for an exciting opportunity in the pharmaceutical industry? Gracure Pharmaceuticals Ltd. is seeking talented individuals to join our team in the field of Drug Regulatory Affairs. As an Executive/Sr. Executive, you will play a crucial role in ensuring regulatory compliance and product life cycle management. If you hold an M. Pharma degree and have 4 to 6 years of experience, we want to hear from you. Read on to discover more about this fantastic career opportunity.
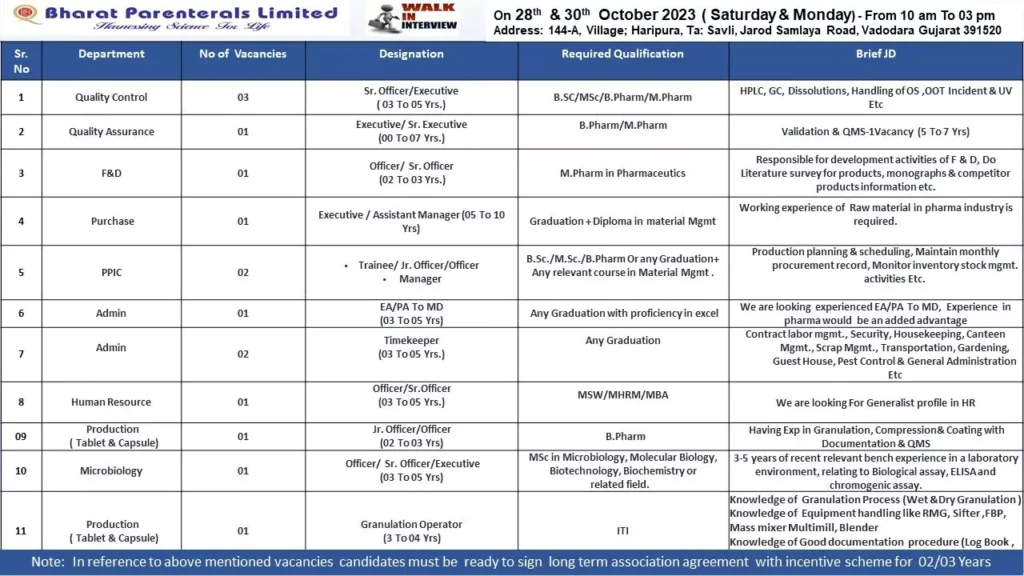
Vacancies List
Company Name: Gracure Pharmaceuticals Ltd.
👉 Never Miss a Pharma Job Again
💼 Join our LIVE WhatsApp Group & Get Instant Updates. 📢 Click below to join:
📱 Join Click HereSalary: Competitive
Company Address: Moti Nagar, New Delhi
Detailed Job Description:
Designation: Senior Executive to Senior Manager
Qualification: M. Pharma
Experience: 6 to 15 years
Industry Type: Pharmaceuticals
Department: Regulatory Affairs
Employment Type: Full-time
Role Category: Regulatory Compliance
Job Description:
- Compiling and reviewing the dossier in accordance with the regulatory requirements of regulated markets (Europe/UK/Canada/Australia).
- In-depth knowledge of regulatory guidelines of major regulatory bodies such as ICH, WHO, EMA, etc.
- Responding to queries from partners and agencies.
- Assessing change control for regulatory adequacy and requirements.
- Ensuring the life cycle management of products to facilitate smooth market supply.
- To ensure the life cycle management of product to smoothen the market supply of product.
Qualifications:
Qualification: M. Pharma
Experience: 06 to 15 years
Key Skills:Regulatory ComplianceDossier CompilationKnowledge of ICH, WHO, EMA GuidelinesChange Control AssessmentLife Cycle Management
Working Location: Moti Nagar, New Delhi
How to Apply:If you are a qualified and passionate professional with the required skills and experience, we encourage you to apply for this position. Please send your CV to:Aakansha Agarwal Email: aakansha.agarwal@gracure.com