Torrent Pharma M.Pharm, MSc Interview: Regulatory Affairs, Formulation

Introduction: Welcome to Torrent Pharma, a distinguished name in the pharmaceutical industry and a proud member of the Torrent Group, one of India’s top pharmaceutical conglomerates. With a strong foothold in therapeutic areas like cardiovascular and central nervous system, we have expanded our presence in various segments including gastro-intestinal, diabetology, anti-infective, and pain management. Torrent Pharma boasts a global presence, operating in over 70 countries across five continents and boasting a remarkable portfolio of over 1200 product registrations.
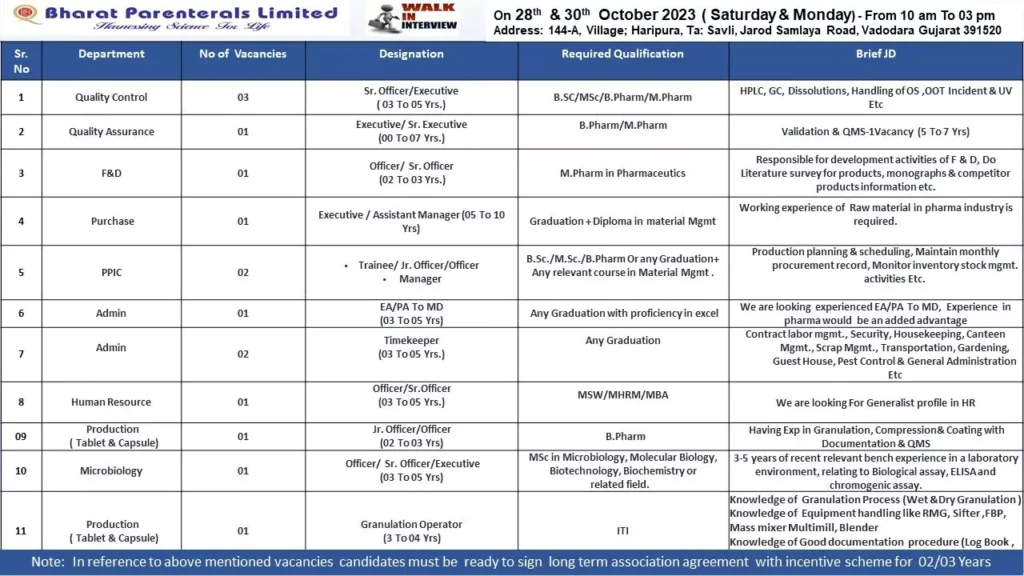
Vacancies List:
- Company Name: Torrent Pharmaceuticals Limited
- Position Titles:
- Regulatory Affairs – Asst Manager
- Regulatory Affairs – Executive
- Formulation Technology Transfer – Executive
- Formulation Development – Executive
- Company Address: Village – Bhat, Near Kanoriya Hospital, Gandhinagar, Gujarat
- Job Type: Full-time
- Location: Gandhinagar, Gujarat
Detailed Job Descriptions:
👉 Never Miss a Pharma Job Again
💼 Join our LIVE WhatsApp Group & Get Instant Updates. 📢 Click below to join:
📱 Join Click HereRole: Regulatory Affairs – Asst Manager
Industry Type: Pharmaceutical
Department: Regulatory Affairs
Employment Type: Full-Time
Role Category: Managerial
Education:
- UG: M.Pharm / MSc.
- Experience: 9 – 12 years
Job Description:
- Compilation and review of registration dossiers following country-specific guidelines.
- Preparation of dossiers in eCTD format for ANDA and EU-MA applications.
- Dossier applications for emerging markets.
- Life cycle management of ANDA, EU-Application, and emerging markets.
- Experience in API DMF filing.
- Preferred experience in solid orals and/or topicals.
Role: Regulatory Affairs – Executive
Industry Type: Pharmaceutical
Department: Regulatory Affairs
Employment Type: Full-Time
Role Category: Executive
Education:
- UG: M.Pharm / MSc.
- Experience: 2 – 8 years
Job Description:
- Compilation and review of registration dossiers following country-specific guidelines.
- Preparation of dossiers in eCTD format for ANDA and EU-MA applications.
- Dossier applications for emerging markets.
- Life cycle management of ANDA, EU-Application, and emerging markets.
- Experience in API DMF filing.
- Preferred experience in solid orals and/or topicals.
Role: Formulation Technology Transfer – Executive
Industry Type: Pharmaceutical
Department: Formulation Technology Transfer
Employment Type: Full-Time
Role Category: Executive
Education:
- UG: M.Pharm
- Experience: 2 – 8 years
Job Description:
- Experience in OSD, Topical, Oncology, Scale up, PDL, PAT, Tech transfer from R&D to Manufacturing locations.
Role: Formulation Development – Executive
Industry Type: Pharmaceutical
Department: Formulation Development
Employment Type: Full-Time
Role Category: Executive
Education:
- UG: M.Pharm
- Experience: 2 – 8 years
Job Description:
- Experience in OSD, Topical, Cosmeceutical, and Oncology.
- Exposure to Regulated, Semi Regulated & India Markets.
Walk-in Interview:
- Date: 28th October 2023 (Saturday)
- Time: 10:00 AM – 5:00 PM
- Venue: Torrent Pharmaceuticals Limited (R&D Centre), Village – Bhat, Near Kanoriya Hospital, Gandhinagar, Gujarat