Dr. Reddy’s Hiring Engineering, Manufacturing, Production, and Quality Control

- Company Overview
- Job Role & Responsibilities
- Engineering Roles
- Production & Packing (Derma)
- Quality Control
- Eligibility / Qualifications
- Location & Interview Details
- Application Process
- FAQs
- Summary Table
Dr. Reddy’s Hiring Team Members | OSD Engineering & QC | Hyderabad
Apply for Team Member roles in Engineering, Production, and QC at Dr. Reddy’s Hyderabad. Openings for Diploma, B.Tech, B.Pharm, B.Sc, M.Sc with 3-7 years’ exp.
👉 Never Miss a Pharma Job Again
💼 Join our LIVE WhatsApp Group & Get Instant Updates. 📢 Click below to join:
📱 Join Click HereDr. Reddy’s Laboratories, a leading multinational pharmaceutical company, is organizing a Career-Expo in Hyderabad for professionals in Engineering, Manufacturing, Production, and Quality Control (QC). This walk-in opportunity is ideal for candidates with strong exposure to USFDA-regulated plants and looking to advance their career with one of India’s most trusted pharma giants.
Company Overview
Dr. Reddy’s Laboratories is a global pharmaceutical leader known for its commitment to affordable and innovative medicines. With a legacy of trust and regulatory excellence, the company provides end-to-end solutions in drug development, manufacturing, and global supply. This career expo reinforces Dr. Reddy’s vision of Good Health Can’t Wait by hiring skilled professionals to strengthen its manufacturing and quality operations.
Job Role & Responsibilities
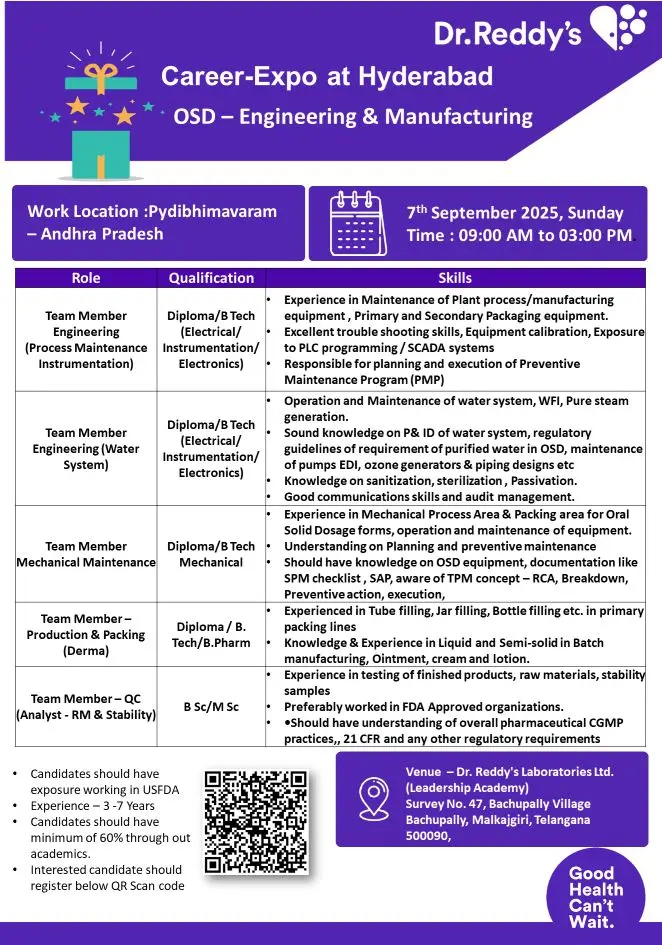
The company is hiring for the following roles at its Pydibhimavaram, Andhra Pradesh facility:
Engineering Roles
- Team Member – Process Maintenance Instrumentation
- Maintenance of plant process/manufacturing equipment.
- Exposure to PLC programming, SCADA systems, and calibration.
- Execution of Preventive Maintenance Programs (PMP).
- Team Member – Water System
- Operation and maintenance of WFI, pure steam generation, EDI, and ozone generators.
- Knowledge of P&ID, water system regulatory guidelines, sanitization, sterilization, and passivation.
- Team Member – Mechanical Maintenance
- Maintenance of mechanical process areas and packaging equipment.
- Knowledge of OSD equipment, preventive maintenance, RCA, TPM concepts.
Production & Packing (Derma)
- Team Member – Production & Packing (Derma)
- Hands-on experience with tube filling, jar filling, and bottle filling in primary packing lines.
- Exposure to liquid and semi-solid dosage manufacturing such as ointments, creams, and lotions.
Quality Control
- Team Member – QC (Analyst – RM & Stability)
- Analysis of raw materials, finished products, and stability samples.
- Proficiency in advanced analytical techniques.
- Compliance with cGMP, 21 CFR, and USFDA regulations.
Eligibility / Qualifications
- Diploma / B.Tech (Electrical, Instrumentation, Electronics, Mechanical).
- B.Pharm / B.Tech / B.Sc / M.Sc (Pharma or Sciences).
- Minimum 3-7 years of experience in relevant functions.
- 60% aggregate required throughout academics.
- Prior exposure to USFDA-regulated plants is mandatory.
Location & Interview Details
- Work Location: Pydibhimavaram, Andhra Pradesh
- Interview Venue: Dr. Reddy’s Laboratories Ltd. (Leadership Academy), Survey No. 47, Bachupally Village, Malkajgiri, Telangana – 500090
- Date & Time: 7th September 2025, Sunday | 09:00 AM – 03:00 PM
Application Process
Interested candidates should register via the QR code provided in the official notification. Only registered candidates will be allowed for the walk-in.
For more details, visit: Dr. Reddy’s Official Careers Page
FAQs
Q1. What is the minimum experience required?
Candidates must have 3–7 years of experience in the pharmaceutical industry with USFDA exposure.
Q2. Can freshers apply?
No, these positions are for experienced professionals only.
Q3. What documents should I bring to the walk-in?
Bring your updated CV, academic certificates, experience letters, last 3 months’ payslips, and ID proof.
Q4. What is the selection process?
Shortlisted candidates will undergo technical interviews followed by HR evaluation.
Q5. Is knowledge of regulatory compliance mandatory?
Yes, familiarity with cGMP, 21 CFR, and USFDA guidelines is essential.
Summary Table
| Company | Dr. Reddy’s Laboratories |
|---|---|
| Vacancies | Multiple – Engineering, Production, QC |
| Required Education | Diploma, B.Tech, B.Pharm, B.Sc, M.Sc |
| Experience | 3–7 Years (USFDA plant exposure mandatory) |
To apply for this job please visit Dr.%20Reddy's%20Official%20Careers%20Page.

