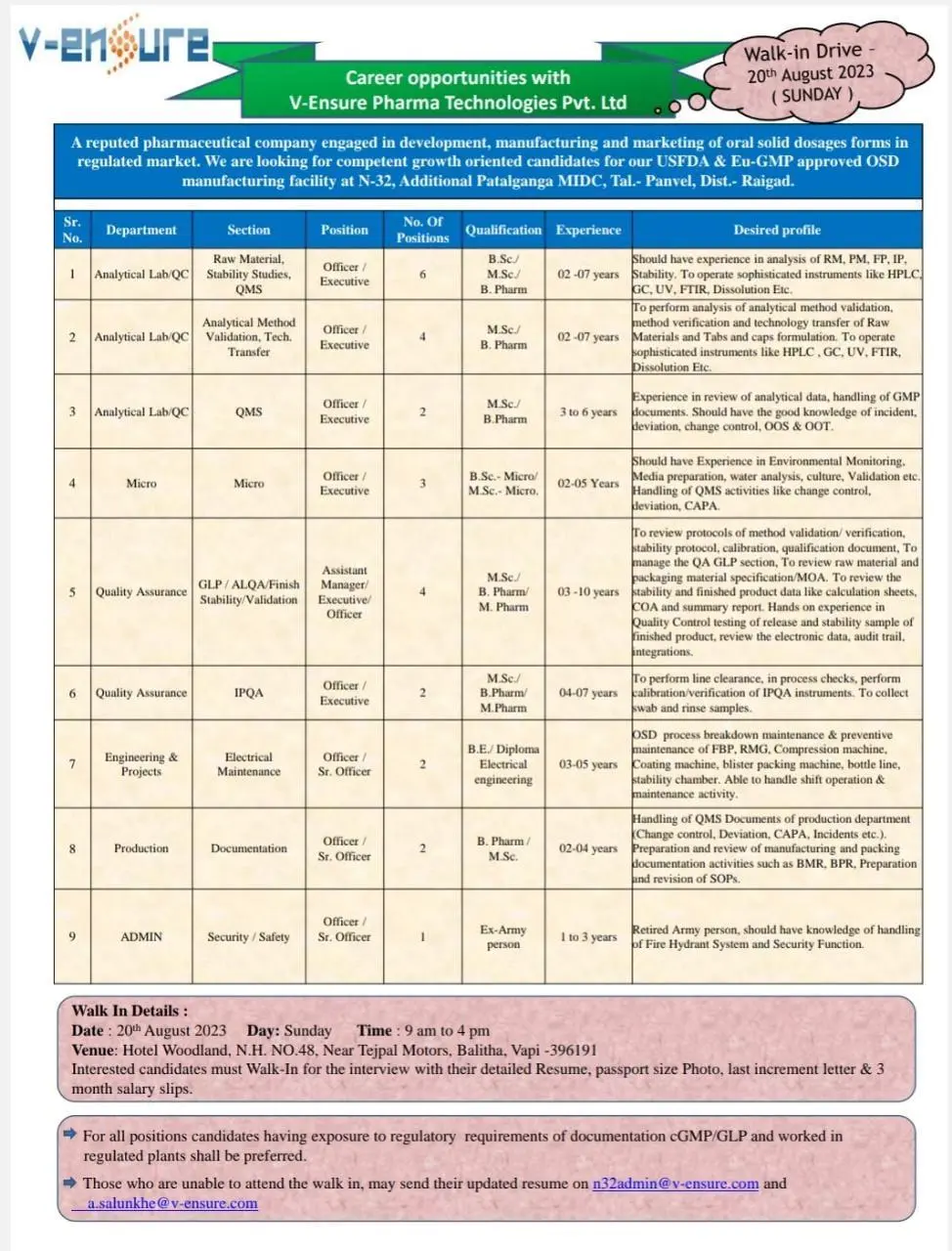
V-Ensure Pharma Technologies Career Opportunities for pharma & chemistry candidates
- Explore Thriving Career Opportunities at V-Ensure Pharma Technologies Pvt. Ltd
- About V-Ensure Pharma Technologies Pvt. Ltd
- Vacancy Details
- Career Opportunities and Qualifications
- 1. Analytical Lab QC (Officer/Executive)
- 2. Analytical Method Validation, Tech. Transfer (Officer/Executive)
- 3. Quality Assurance (Assistant Manager/Executive/Officer)
- 4. Micro (Officer/Executive)
- 5. Engineering & Projects (Officer/Sr. Officer)
- 6. Production Documentation (Officer/St. Officer)
- 7. Security/Safety (Ex-Army Person)
- Walk-in Drive
Explore Thriving Career Opportunities at V-Ensure Pharma Technologies Pvt. Ltd
V-Ensure Pharma Technologies Pvt. Ltd, a distinguished pharmaceutical company engaged in the development, manufacturing, and marketing of oral solid dosage forms in regulated markets, is offering promising career opportunities. If you are a growth-oriented candidate seeking a fulfilling career in the pharmaceutical industry, seize the chance to be a part of their journey by attending their Walk-in Drive on August 20th, 2023 (Sunday).
About V-Ensure Pharma Technologies Pvt. Ltd
V-Ensure Pharma Technologies Pvt. Ltd stands as a prominent player in the pharmaceutical sector, specializing in the development, manufacturing, and marketing of oral solid dosage forms. With a reputation for quality and innovation, the company operates within the framework of regulatory compliance to ensure that its products meet the highest standards.
Vacancy Details
Career Opportunities and Qualifications
V-Ensure Pharma Technologies Pvt. Ltd is offering positions in various departments, each requiring specific qualifications and experience levels. Here are some of the opportunities available:
1. Analytical Lab QC (Officer/Executive)
- Qualification: BSc/MSc/B.Pharm
- Experience: 2-7 years (MSc), 2-7 years (Ph.D)
- Responsibilities: Conduct analysis of Raw Materials (RM), Packaging Materials (PM), Finished Products (FP), Intermediate Products (IP), and Stability. Operate sophisticated instruments such as HPLC, GC, UV, FTIR, Dissolution, etc.
2. Analytical Method Validation, Tech. Transfer (Officer/Executive)
- Qualification: MSc/B.Pharm
- Experience: 2-7 years
- Responsibilities: Perform analysis, validation, verification, and technology transfer of Raw Materials (RM) and Tablets/Capsules formulations. Operate HPLC, GC, UV, FTIR, Dissolution, etc.
3. Quality Assurance (Assistant Manager/Executive/Officer)
- Qualification: MSc/B.Pharm/M.Pharm
- Experience: 3-10 years
- Responsibilities: Review protocols of method validation, stability, calibration, qualification documents. Manage QA GLP section, review raw material and packaging material specifications, review stability and finished product data.
4. Micro (Officer/Executive)
- Qualification: BSc-Micro/MSc-Micro
- Experience: 2-5 years
- Responsibilities: Environmental Monitoring, Media Preparation, Water Analysis, Culture, Validation, etc. Handle QMS activities like change control, deviation, CAPA.
5. Engineering & Projects (Officer/Sr. Officer)
- Qualification: B.E/Diploma Electrical Engineering
- Experience: 3-5 years
- Responsibilities: Maintenance of OSD process equipment, handling shift operations & maintenance activities.
6. Production Documentation (Officer/St. Officer)
- Qualification: B.Pharm/MSc
- Experience: 2-4 years
- Responsibilities: Handle QMS documents of the production department, preparation and review of manufacturing and packing documentation.
7. Security/Safety (Ex-Army Person)
- Experience: 1-3 years
- Responsibilities: Handling of Fire Hydrant System and Security Functions.
Walk-in Drive
👉 Never Miss a Pharma Job Again
💼 Join our LIVE WhatsApp Group & Get Instant Updates. 📢 Click below to join:
📱 Join Click HereV-Ensure Pharma Technologies Pvt. Ltd is seeking competent and driven individuals to join their team. The Walk-in Drive details are as follows:
Date: August 20th, 2023
Day: Sunday
Time: 9 am to 4 pm
Venue: Hotel Woodland, N.H. NO.48, Near Tejpal Motors, Balitha, Vapi – 396191
Interested candidates are encouraged to attend the interview with their detailed resume, passport-sized photo, last increment letter, and 3-month salary slips.