Macleods Walk-in 20+ vacancies in R&D, Regulatory Affairs, PDR

- Company Overview
- Job Role & Responsibilities
- 1. Sr. Research Associate / Research Scientist – PDR
- 2. Sr. Executive / Executive – Regulatory Affairs (US, Europe, South Africa, Africa, AU, NZ & Canada)
- 3. Sr. Officer / Officer – Regulatory Affairs (US, Europe, AU, NZ, CIS, LATAM & MENA)
- Eligibility / Qualifications
- Location & Walk-in Details
- Application Process
- Why Join Macleods?
- FAQs
- Summary Table
M.Pharm, PhD, MSc Vacancies | Walk-in Drive at Macleods R&D Mumbai
Macleods Walk-in Drive at Mumbai for M.Pharm, PhD, MSc candidates | 20+ vacancies in R&D, Regulatory Affairs, PDR | Apply on 13 Sept 2025.
👉 Never Miss a Pharma Job Again
💼 Join our LIVE WhatsApp Group & Get Instant Updates. 📢 Click below to join:
📱 Join Click HereMacleods Pharmaceuticals, a globally trusted pharmaceutical company, is conducting a Walk-in Drive in Mumbai for its R&D and Regulatory Affairs departments. With an impressive presence in over 140 countries, Macleods is known for its innovative formulations, strict adherence to global regulatory standards, and commitment to healthcare advancement. This recruitment drive offers excellent opportunities for experienced professionals in research and development, regulatory affairs, and product development.
Company Overview
Macleods Pharmaceuticals Ltd. is one of India’s fastest-growing pharmaceutical companies, recognized for its strong R&D foundation and regulatory compliance. With multiple state-of-the-art facilities approved by USFDA, EMA, and WHO, Macleods focuses on delivering quality medicines across global markets.
Job Role & Responsibilities
1. Sr. Research Associate / Research Scientist – PDR
- Conduct formulation and development of solid oral dosage forms and liquid orals.
- Manage pre-formulation studies, scale-up, and technology transfer.
- Ensure compliance with regulatory and quality guidelines for global markets.
2. Sr. Executive / Executive – Regulatory Affairs (US, Europe, South Africa, Africa, AU, NZ & Canada)
- Prepare and review CTD dossiers, ANDAs, and regulatory submissions.
- Respond to regulatory queries and manage post-approval variations.
- Liaise with global regulatory agencies to ensure compliance.
3. Sr. Officer / Officer – Regulatory Affairs (US, Europe, AU, NZ, CIS, LATAM & MENA)
- Handle product lifecycle management for assigned markets.
- Prepare and submit regulatory dossiers, respond to deficiency letters.
- Manage post-approval changes and documentation.
Eligibility / Qualifications
- Education: M.Pharm, PhD (Pharmaceutics), MSc (Life Sciences, Chemistry, Pharmaceutical Sciences)
- Experience:
- PDR: 4–9 years in solid oral dosage & liquid orals (F&D).
- Sr. Executive/Executive RA: 6–11 years in global regulatory affairs.
- Sr. Officer/Officer RA: 2–5 years in dossier preparation and lifecycle management.
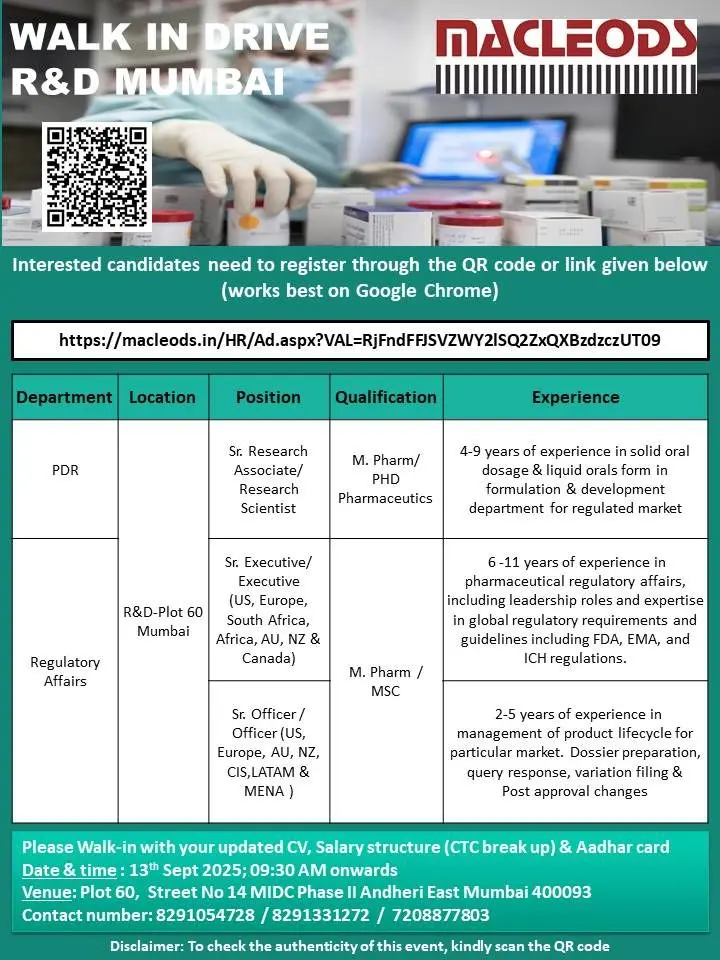
Location & Walk-in Details
- Date & Time: 13th September 2025 | 09:30 AM onwards
- Venue: Plot 60, Street No. 14, MIDC Phase II, Andheri East, Mumbai – 400093
- Contact Numbers: 8291054728 / 8291331272 / 7208877803
Application Process
- Step 1: Register through the official Macleods career portal or scan the QR code in the official announcement.
- Step 2: Attend the walk-in with the following documents:
- Updated CV
- Salary structure (CTC breakup)
- Aadhar card
Apply before 13th September 2025 and secure your chance to be part of a leading global pharmaceutical company!
Why Join Macleods?
- Exposure to regulated markets (USFDA, EMA, WHO, etc.)
- Career growth in global R&D and Regulatory Affairs
- Collaborative environment fostering innovation
- Competitive salary and benefits package
FAQs
Q1: Who can apply for these roles?
Candidates with M.Pharm, PhD (Pharmaceutics), or MSc with 2–11 years of relevant experience in R&D and Regulatory Affairs can apply.
Q2: Is prior experience in regulated markets mandatory?
Yes, candidates with exposure to USFDA/EMA/ICH regulations are preferred.
Q3: What documents should I carry for the interview?
Updated CV, salary breakup, Aadhar card, and educational certificates.
Q4: Can I apply online if I cannot attend the walk-in?
Yes, candidates can register and apply through the official career link provided.
Summary Table
| Company | Macleods Pharmaceuticals Ltd. |
|---|---|
| Vacancies | Sr. Research Associate, Research Scientist, Sr. Executive, Executive, Sr. Officer, Officer |
| Required Education | M.Pharm, PhD (Pharmaceutics), MSc |
| Experience | 2–11 Years (depending on role) |
To apply for this job please visit macleods.in.

