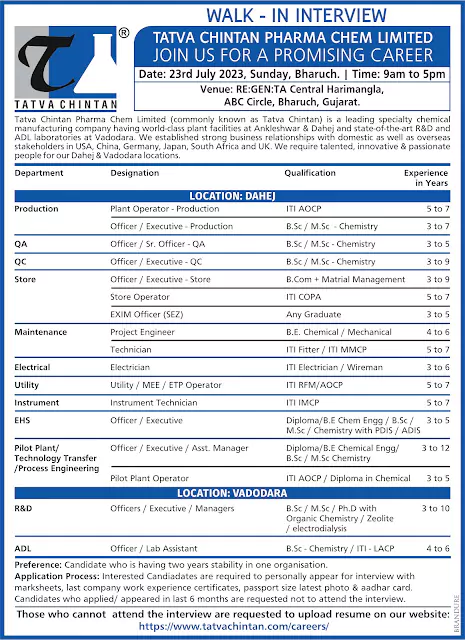
Ipca Laboratories Walk in interview at Ratlam

- Ipca Laboratories Vacancies information :
- Quality Assurance (Formulation)
- Injection/Injectable, Formulation
- Engineering, Pharma (Maintenance)
- Quality Control (API)
- Quality Control (Formulation)
- WALK IN INTERVIEW Details :
Ipca Laboratories walk in interview for Quality Assurance, Injection/Injectable, Formulation, Engineering, Pharma (Maintenance), Quality Control (Formulation), Quality Control (API)
Ipca Laboratories Vacancies information :
Quality Assurance (Formulation)
Designation : Executive/ Sr. Officer/ Officer
Experience : 3 to 10 years
Educational Qualification : BSc / MSc / B Pharma / M Pharma
Expertise : IPQA, Sterile: IPQA, Qualification, Validation, Analytical QA, Stability Studies, regulatory affairs, OOS investigation (Microbiological), Risk Assessment, Market Complaint, Documentation, Exposure in QMS, Deviation, CAPA etc with Trackwise system.
Injection/Injectable, Formulation
position : Executive/ Sr Officer/ Officer
Experience : 3 to 10 years
Educational Qualification : BSc./MSc./B.Pharma/ M.Pharma
Skills Required : Sterile area manufacturing (Ampoule and Vial) Shift supervision, Knowledge of OOS, OOT, CAPA, online documentation as per SOP/BMR. Instruments/equipments like: Autoclave, Washing, Filling and sealing Machine etc. and concern activities related to batch Manufacturing, filling, labeling, cartoning, 2D serialization, validation activities like media preparation, media filling.
Engineering, Pharma (Maintenance)
👉 Never Miss a Pharma Job Again
💼 Join our LIVE WhatsApp Group & Get Instant Updates. 📢 Click below to join:
📱 Join Click HereDesignation : Executive/ Sr. Officer/ Officer
Experience :4 to 8 year
Education Required : BE/B.Tech/ Diploma
Job Skills ; Require engineer for break down & trouble shooting at vial & ampoule line equipments of injectable facility, handling maintenance of machines like Vial washing, Vial Filling & sealing machine, Rotary washing, Filling & Sealing, inspection & Packing machines autoclave, Have exposure of trouble shooting of equipments knowledge of breakdown related to the tablet, liquid & injectable equipments.
Quality Control (API)
Designation : Sr. Officer/ Officer
Experience : 2 to 5 years
Education Required : BSc./B.Pharma
Job Responsibilities : Required Analyst/ Reviewer for API products analysis using for Stability, Raw Material, Finished product & In-process lab Instruments like: HPLC/ GC/LCMS operations, Calibration & Validation related testing like: Validation, Calibration, Temperature & Environmental Monitoring. Also expertise in Stability Sample Management, Protocol Preparation, QMS (OOS, OOT & Incident Investigation)
Quality Control (Formulation)
Designation : Executive/Sr. Officer/ Officer
Experience : 2 to 10 years
Education Required : BSc./B.Pharma
Job Skills : Require Analyst/ Reviewer for Formulation products (i.e. Injection, Tablet, Dry Syrup, Liquid) analysis using for Wet lab/ Chemical/Stability & Micro lab with core exposure of instruments like HPLC/ GC/ UV/IR operations & Micro related testing
(Experience. required from Sterile / Injection Micro) like Microbiology Lab, MLT, Sterility of injectable, Bacterial Endotoxin Test (BET), Media Preparation, Validation protocol Validation of Sterility Test, Reconciliation & Water Analysis, Temperature & Environmental Monitoring. Also expertise in Stability Sample Management, Protocol Preparation, Chromatography Integration, LIMS, GLP, GDP, QMS (OOS, OOT & Incident Investigation).
Note: Candidates may share CV on email: vikas.rao@ipca.com, pragya.vvas@ipca.com, aishwarya.tomar@ipca.com, vishal.sharma@ipca.com, nikita.chouhan@ipca.com, chandni.verma@ipca.com, Contact Number: 07412-27/8462/8079/8089/8358
WALK IN INTERVIEW Details :
Date: 20th Nov., 2022 (Sunday), Time: 10:00 am to 5:00 pm
Venue : “Hotel KC CROSS ROAD” Site No 1, Sector 10, Opp. Bus Stand Panchkula- (HARYANA)
Location :Ipca Laboratories Limited, Location: Ratlam (M.P.)
Note: “MSc.” Qualified Candidates will not be entertained in Walk-In Interview for QC department.