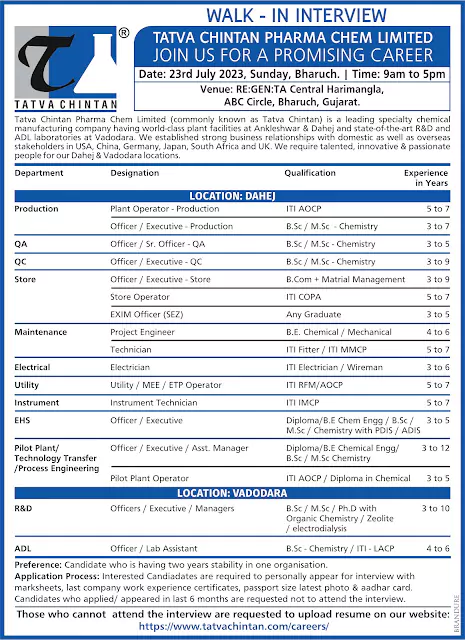
Zydus Healthcare Limited Hiring for EHS, production, warehouse, production, Engineering, Quality control Departments

Zydus Healthcare Limited Inviting candidates for a walk-in interview for Manufacturing & Quality functions for OSD & injectable manufacturing facility at Daman on 11th June, Sunday from 10 am to 4 pm.
Injectable Production
Plant Operator / Technical Assistant: ITI / D.Pharm / Diploma with 1-6 years of relevant experience in operating production machines like autoclave, vial filling, vial sterilization tunnel, vial sealing & capping, hi-cart, visual inspector for dry powder injection vial etc.
👉 Never Miss a Pharma Job Again
💼 Join our LIVE WhatsApp Group & Get Instant Updates. 📢 Click below to join:
📱 Join Click HereOfficer / Executive: B Pharmacy / M Pharm with 2-6 years of experience in an injectable manufacturing plant. Candidates should have knowledge of cGMP, manufacturing & packing.
Production
Officer / Executive: B Pharmacy / M Pharm with 3-6 years of experience in OSD & injectable production – QMS, change control, deviation and investigation. Candidates should have exposure to regulatory audits.
Engineering
Officer / Executive B. E / B Tech with 4-7 years of work experience in pharmaceutical company, handling of electrical process and utility maintenance, HVAC and hands on experience in handling engineering QMS documentations.
Plant Operator / Technical Assistant: ITI / Diploma (Electrical / Mechanical) with 1-6 years of work experience in pharmaceutical company, handling of electrical process and utility maintenance.
Quality Control
Officer / Executive BSC / MSC / B Pharmacy / M. Pharm with 2-6 years of experience in analysis / finish / stability b / sampling by using instruments like HPLC, UV-visible spectrophotometer, dissolution apparatus, GC chromatography. IR spectrophotometer and should have exposure to regulatory audits.
Quality Control – Micro Section
Officer / Executive: BSC / MSC with 2-6 years of experience in media preparation, culture handling. MLT / sterility / BET testing by using instruments like LPC, autoclave and should have exposure to regulatory audits.
Quality Control – CSV Engineer
Officer / Executive: BSC / MSC / B. E / B Tech with 2-6 years of experience in
comprehensive knowledge in FDA’s 21 CFR Part 11 regulations, EU Annex 11, GAMP Software categorization, good understanding in CSV processes and preparation of validation/qualification documents as per VMP.
Warehouse
Officer / Executive: B Pharmacy / M Pharm / BSC / MSC / B Com / M Com with 3-6 years of experience in warehouse handling of raw material and packing material dispensing. Candidates should have exposure to regulatory audits.
EHS
Executive / Sr Executive: BSC / MSC / B.E / M.E with 5-9 years of experience in EHS in handling all the safety, health and environmental activities and of which candidates should have a minimum of 2 years of experience in handling EHS functions independently in a manufacturing plant
For all the positions mentioned, candidates should have exposure to working in an OSD & Injectables setup and regulatory requirements of documentation as per cGMP/GDP. They can walk-in with their updated CVs, passport size photo and latest annual CTC breakup details and can also share their updated CVs with satish.singh@zyduslife.com.
Please note that Zydus does not hire consultants/agents who promise interviews / jobs for monetary consideration / registration fees. Beware of such fraudulent calls.
Venue : Zydus Healthcare Limited, Survey No. 49/3, 51/1, 51/2, Ringanwada Village, Daman – 396 210