Ciron Drugs & Pharmaceuticals Walk-in QC, QA, Production, Warehouse, Engineering

- Company Overview
- Job Role & Responsibilities
- 1. Quality Control (QC) – HPLC/Stability
- 2. Quality Assurance (QA) – Validation / Qualification
- 3. Quality Assurance (QA) – Lab QA
- 4. Production – Granulation/Compression/Coating
- 5. Production – QMS
- 6. Production – Packing
- 7. Production – Capsule & Dry Syrup
- 8. Production – Liquid (QMS)
- 9. Production – Liquid/External Filling & MFG
- 10. Warehouse – RM/PM
- 11. Warehouse – BSR
- 12. Engineering – ETP
- Eligibility / Qualifications
- Location & Salary
- Application Process
- Why Join Ciron Drugs?
- FAQs
- Summary Table
B.Pharm/M.Sc Vacancies – QC, QA, Production at Ciron Palghar
Ciron Drugs hiring in QC, QA, Production & Warehouse. Walk-in on 6th–7th Sept 2025 at Palghar. Vacancies for B.Pharm, M.Sc, B.Sc, Diploma candidates.
👉 Never Miss a Pharma Job Again
💼 Join our LIVE WhatsApp Group & Get Instant Updates. 📢 Click below to join:
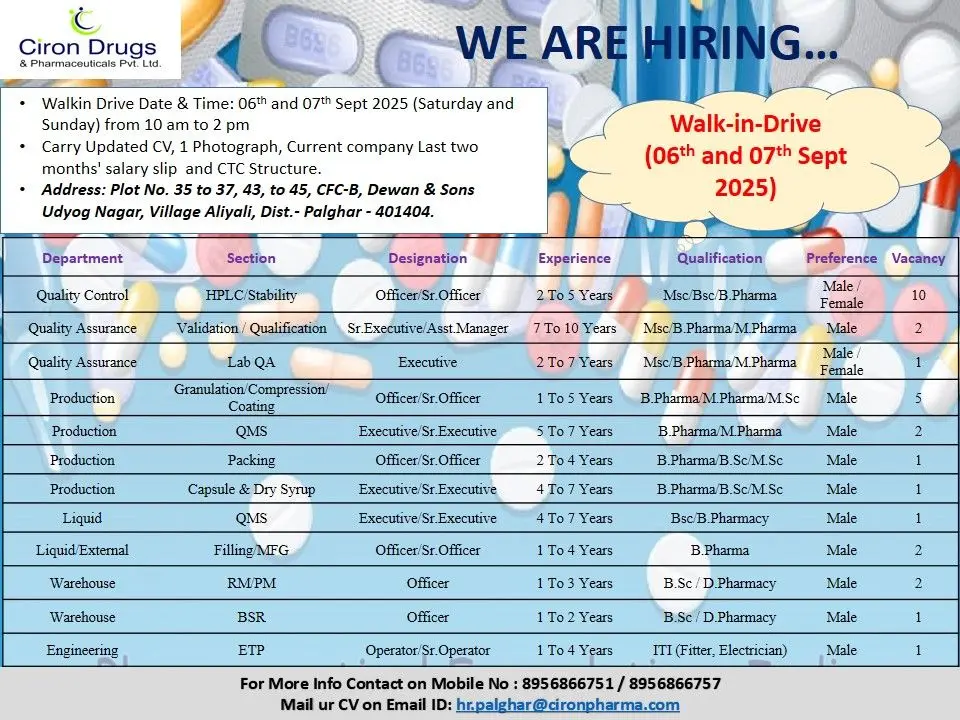
📱 Join Click HereAre you seeking a rewarding career in the pharmaceutical industry? Ciron Drugs & Pharmaceuticals Pvt. Ltd., a trusted name in quality formulations, is conducting a walk-in drive on 6th & 7th September 2025 at Palghar (Maharashtra). With multiple vacancies across Quality Control, Quality Assurance, Production, Warehouse, and Engineering, this is a golden opportunity for experienced pharma professionals to join one of India’s leading healthcare organizations.
Company Overview
Ciron Drugs & Pharmaceuticals Pvt. Ltd. is a well-established pharmaceutical company recognized for its global presence in formulations, injectables, and APIs. With WHO-GMP certified facilities and a strong emphasis on regulatory compliance, Ciron Drugs consistently delivers high-quality medicines across therapeutic areas.
For over decades, the company has built trust with healthcare providers and patients worldwide by ensuring innovation, affordability, and reliability in its pharmaceutical products.
Job Role & Responsibilities
1. Quality Control (QC) – HPLC/Stability
Positions: Officer / Sr. Officer
Experience: 2–5 Years
Qualification: M.Sc / B.Sc / B.Pharm
Responsibilities: Conduct HPLC and stability studies, ensure compliance with GLP and cGMP, manage documentation, and support audits.
2. Quality Assurance (QA) – Validation / Qualification
Positions: Sr. Executive / Asst. Manager
Experience: 7–10 Years
Qualification: M.Sc / B.Pharm / M.Pharm
Responsibilities: Oversee equipment validation, process qualification, ensure QMS compliance, and lead regulatory audit readiness.
3. Quality Assurance (QA) – Lab QA
Position: Executive
Experience: 2–7 Years
Qualification: M.Sc / B.Pharm / M.Pharm
Responsibilities: Manage lab QA activities, review records, ensure GLP compliance, and support laboratory documentation.
4. Production – Granulation/Compression/Coating
Positions: Officer / Sr. Officer
Experience: 1–5 Years
Qualification: B.Pharm / M.Pharm / M.Sc
Responsibilities: Oversee solid oral dosage manufacturing processes, monitor BMR/BPR, ensure GMP adherence, and troubleshoot process issues.
5. Production – QMS
Positions: Executive / Sr. Executive
Experience: 5–7 Years
Qualification: B.Pharm / M.Pharm
Responsibilities: Handle QMS-related production activities, deviation management, CAPA, and change control processes.
6. Production – Packing
Positions: Officer / Sr. Officer
Experience: 2–4 Years
Qualification: B.Pharm / B.Sc / M.Sc
Responsibilities: Operate and monitor blister, bottle, and bulk packing lines, ensure compliance with SOPs, and maintain packing records.
7. Production – Capsule & Dry Syrup
Positions: Executive / Sr. Executive
Experience: 4–7 Years
Qualification: B.Pharm / B.Sc / M.Sc
Responsibilities: Manage capsule filling and dry syrup manufacturing, ensure process validation, and maintain GMP compliance.
8. Production – Liquid (QMS)
Positions: Executive / Sr. Executive
Experience: 4–7 Years
Qualification: B.Sc / B.Pharm
Responsibilities: Oversee QMS activities in liquid manufacturing, review deviations, support CAPA, and process improvements.
9. Production – Liquid/External Filling & MFG
Positions: Officer / Sr. Officer
Experience: 1–4 Years
Qualification: B.Pharm
Responsibilities: Manage filling operations for liquid/external preparations, conduct in-process checks, and ensure adherence to SOPs.
10. Warehouse – RM/PM
Position: Officer
Experience: 1–3 Years
Qualification: B.Sc / D.Pharmacy
Responsibilities: Handle receipt, storage, and dispensing of Raw Materials (RM) and Packaging Materials (PM).
11. Warehouse – BSR
Position: Officer
Experience: 1–2 Years
Qualification: B.Sc / D.Pharmacy
Responsibilities: Manage Batch Storage Records (BSR), ensure traceability, and maintain inventory documentation.
12. Engineering – ETP
Positions: Operator / Sr. Operator
Experience: 1–4 Years
Qualification: ITI (Fitter / Electrician)
Responsibilities: Operate Effluent Treatment Plant (ETP), conduct preventive maintenance, and ensure environmental compliance.
Eligibility / Qualifications
Degrees Accepted: B.Pharm, M.Pharm, M.Sc, B.Sc, D.Pharm, ITI (Fitter/Electrician)
Experience Range: 1 to 10 years depending on role
Key Skills: cGMP, QMS, HPLC, Stability Studies, Validation, BMR/BPR review, Packing operations, Warehouse management, ETP operations
Location & Salary
Interview Venue & Work Location:
Ciron Drugs & Pharmaceuticals Pvt. Ltd.
Plot No. 35–37, 43–45, CFC-B, Dewan & Sons Udyog Nagar, Village Aliyali, Dist. Palghar – 401404, MaharashtraInterview Dates & Time:
6th & 7th September 2025 (Saturday & Sunday)
10:00 AM to 2:00 PMSalary: Competitive package based on qualifications and experience (Best in Industry).
Application Process
Steps to Apply:
Attend the walk-in drive with:
Updated CV
One passport size photograph
Last 2 months’ salary slips
Current CTC structure
Educational and experience certificates
For candidates unable to attend:
Send your CV to hr.palghar@cironpharma.com
For more details, contact: +91 8956866751 / +91 8956866757
Why Join Ciron Drugs?
Work with a WHO-GMP approved pharma company with global market presence.
Exposure to regulatory audits and international standards.
Career growth across QC, QA, Production, and Engineering.
Opportunity to contribute to high-quality healthcare solutions.
FAQs
Q1: Who can attend this walk-in drive?
Candidates with relevant degrees (B.Pharm, M.Pharm, M.Sc, B.Sc, D.Pharm, ITI) and 1–10 years of experience in pharma manufacturing.
Q2: Are freshers eligible?
This drive is for experienced candidates (minimum 1 year).
Q3: What documents should I bring?
Bring CV, photo, last 2 months’ payslips, CTC details, and academic/experience certificates.
Q4: Where is the interview location?
At Ciron Drugs, Village Aliyali, Palghar, Maharashtra.
Q5: Is there any fee for applying?
No, Ciron Drugs never charges candidates for recruitment.
Summary Table
To apply for this job email your details to hr.palghar@cironpharma.com