BioClinAarc Hiring Opportunities for Clinical Research Professionals in Nagpur
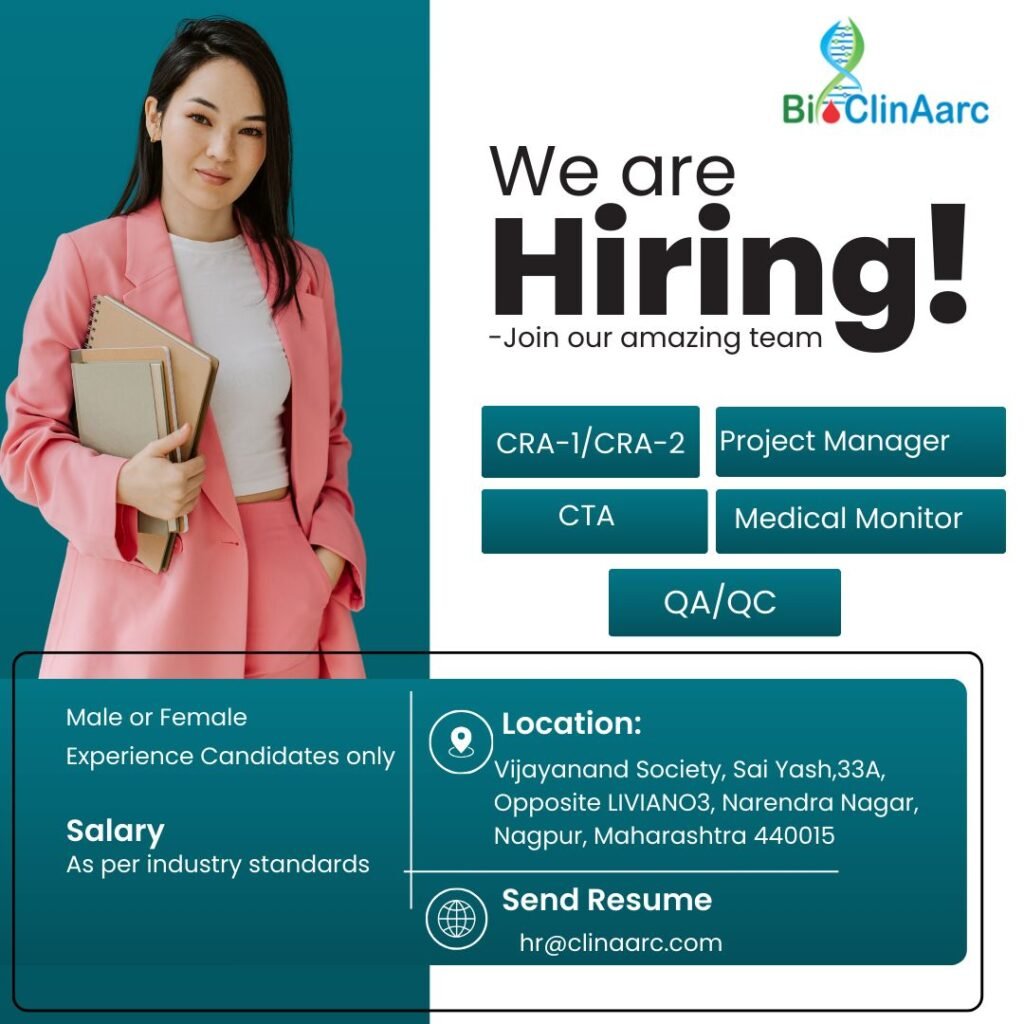
- BioClinAarc Hiring for Clinical Research Roles in Nagpur – Apply Now!
- Why Join BioClinAarc?
- Current Job Openings at Bi ClinAarc
- 1. Clinical Research Associate (CRA-1 & CRA-2)
- 2. Project Manager
- 3. Clinical Trial Assistant (CTA)
- 4. Medical Monitor
- 5. Quality Assurance (QA/QC)
- Job Location & Salary Details
- How to Apply?
- Summary Table for Quick Reference
BioClinAarc Hiring for Clinical Research Roles in Nagpur – Apply Now!
Are you an experienced clinical research professional looking for your next career move? Bi ClinAarc, a leading organization in clinical research, is actively hiring for multiple positions at its Nagpur location. If you have expertise in clinical research and want to work in a dynamic and innovative environment, this opportunity is for you!
Why Join BioClinAarc?
- Work with industry experts in clinical research and regulatory affairs.
- Competitive salary packages as per industry standards.
- Opportunity to grow in a well-established clinical research organization.
- Located in Nagpur, offering a great work-life balance.
Current Job Openings at Bi ClinAarc
1. Clinical Research Associate (CRA-1 & CRA-2)
Responsibilities:
- Conduct and monitor clinical trials as per regulatory guidelines.
- Ensure compliance with protocols, SOPs, and GCP standards.
- Maintain accurate documentation and data collection.
👉 Never Miss a Pharma Job Again
💼 Join our LIVE WhatsApp Group & Get Instant Updates. 📢 Click below to join:
📱 Join Click HereQualifications & Requirements:
- Bachelor’s or Master’s degree in Pharmacy, Life Sciences, Biotechnology, or a related field.
- Minimum 1-3 years of experience in clinical research.
- Strong knowledge of ICH-GCP guidelines.
2. Project Manager
Responsibilities:
- Oversee and manage clinical research projects from initiation to completion.
- Coordinate with internal teams, sponsors, and regulatory authorities.
- Ensure adherence to project timelines and budgets.
Qualifications & Requirements:
- Bachelor’s or Master’s degree in Pharmacy, Life Sciences, Biotechnology, or Clinical Research.
- Minimum 5+ years of experience in project management within the clinical research industry.
- Strong leadership and communication skills.
3. Clinical Trial Assistant (CTA)
Responsibilities:
- Support clinical research teams in documentation and coordination.
- Maintain regulatory files and ensure compliance with guidelines.
- Assist in site management and data entry.
Qualifications & Requirements:
- Bachelor’s degree in Life Sciences, Biotechnology, or Pharmacy.
- 1-2 years of experience in clinical trials or research coordination.
- Strong organizational and multitasking skills.
4. Medical Monitor
Responsibilities:
- Provide medical oversight for clinical trials.
- Review and analyze clinical trial data.
- Ensure safety and compliance with medical standards.
Qualifications & Requirements:
- MBBS or MD degree with specialization in pharmacology, medicine, or a related field.
- 3-5 years of experience in clinical research or drug safety monitoring.
- Strong understanding of clinical protocols and medical regulations.
5. Quality Assurance (QA/QC)
Responsibilities:
- Ensure compliance with clinical research quality standards.
- Conduct audits and inspections for regulatory adherence.
- Implement and monitor quality improvement programs.
Qualifications & Requirements:
- Bachelor’s or Master’s degree in Pharmacy, Life Sciences, or Clinical Research.
- 2-4 years of experience in clinical research quality assurance.
- Strong knowledge of GCP and regulatory guidelines.
Job Location & Salary Details
📍 Location: Vijayanand Society, Sai Yash, 33A, Opposite LIVIANO3, Narendra Nagar, Nagpur, Maharashtra 440015 💰 Salary: Competitive, as per industry standards.

How to Apply?
Interested candidates can send their updated resumes to hr@clinaarc.com with the subject line “Application for [Job Title] – Bi ClinAarc.”
Summary Table for Quick Reference
| Company Name | Current Vacancies | Required Education | Experience Required |
|---|---|---|---|
| BioClinAarc | CRA-1, CRA-2 | B.Pharm, M.Pharm, Life Sciences, Biotechnology | 1-3 years |
| BioClinAarc | Project Manager | B.Pharm, M.Pharm, Life Sciences, Biotechnology | 5+ years |
| BioClinAarc | CTA | B.Sc, M.Sc, Pharmacy, Life Sciences | 1-2 years |
| BioClinAarc | Medical Monitor | MBBS, MD | 3-5 years |
| BioClinAarc | QA/QC | B.Pharm, M.Pharm, Life Sciences | 2-4 years |
To apply for this job email your details to hr@clinaarc.com

