USV Pharma Walk-in QA, QC, Production, Maintenance

- Company Overview
- Job Role & Responsibilities
- Quality Department Openings
- Operations & Maintenance Department Opening
- Eligibility / Qualifications
- Location & Salary
- Application Process
- Why Join USV Pharma?
- FAQs
- Job Summary Table
USV Pharma Walk-in Drive – QA, QC, Production, Maintenance Daman
USV Pvt Ltd hiring B.Pharm/M.Pharm/M.Sc/ITI/Diploma candidates with 2–10 yrs exp in QA, QC, Production & Maintenance. Walk-in Daman on 14 Sept 2025.
👉 Never Miss a Pharma Job Again
💼 Join our LIVE WhatsApp Group & Get Instant Updates. 📢 Click below to join:
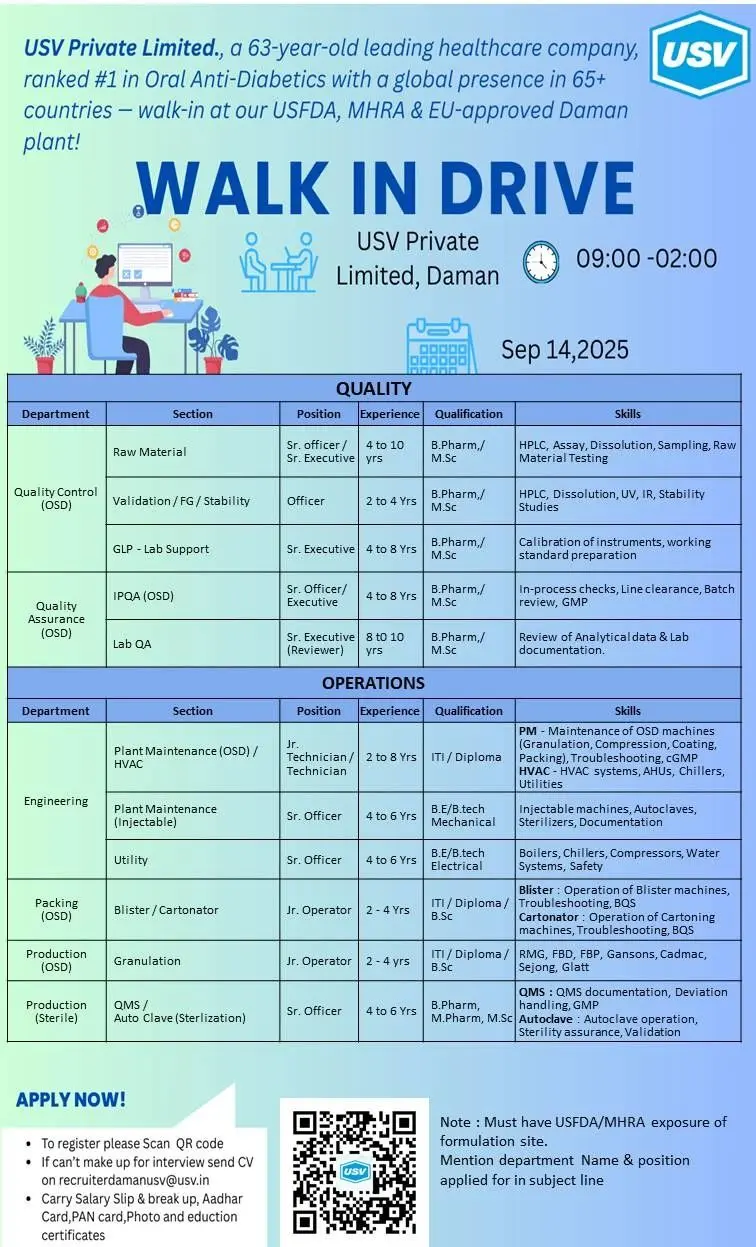
📱 Join Click HereUSV Private Limited, a 63-year-old leading healthcare company and the #1 player in Oral Anti-Diabetics, is conducting a Walk-in Drive at its USFDA, MHRA & EU-approved plant in Daman. With a strong global presence in 65+ countries, USV offers an exciting opportunity for experienced professionals in Quality Assurance, Quality Control, Production, and Plant Maintenance.
Company Overview
USV Pvt. Ltd. is a trusted name in Indian pharmaceuticals with over six decades of excellence in healthcare. The company is known for its regulatory-compliant facilities and robust product portfolio in Oral Anti-Diabetics, Cardiovascular, Biosimilars, Injectables, and API manufacturing. With a strong international footprint, USV provides employees with exposure to regulated markets like the US, EU, and UK, making it a preferred choice for pharma professionals.
Job Role & Responsibilities
The walk-in drive is open for multiple roles across QA, QC, Production, and Maintenance functions at the Daman formulation site.
Quality Department Openings
- Raw Material – Sr. Officer/Sr. Executive
- Experience: 4–10 years
- Qualification: B.Pharm / M.Sc
- Skills: HPLC, Assay, Dissolution, Sampling, Raw Material Testing
- Quality Control (OSD – Validation/FG/Stability) – Officer
- Experience: 2–4 years
- Qualification: B.Pharm / M.Sc
- Skills: HPLC, Dissolution, UV, IR, Stability Studies
- GLP – Lab Support – Sr. Executive
- Experience: 4–8 years
- Qualification: B.Pharm / M.Sc
- Skills: Instrument calibration, working standard preparation
- Quality Assurance (OSD – IPQA) – Sr. Officer/Executive
- Experience: 4–8 years
- Qualification: B.Pharm / M.Sc
- Skills: In-process checks, Line clearance, Batch review, GMP compliance
- Lab QA – Sr. Executive (Reviewer)
- Experience: 8–10 years
- Qualification: B.Pharm / M.Sc
- Skills: Review of analytical data & lab documentation
Operations & Maintenance Department Opening
- Plant Maintenance (OSD/HVAC) – Jr. Technician/Technician
- Experience: 2–8 years
- Qualification: ITI/Diploma Engineering
- Skills: Maintenance of OSD machines, cGMP troubleshooting, HVAC systems
- Plant Maintenance (Injectables) – Sr. Officer
- Experience: 4–6 years
- Qualification: B.E/B.Tech Mechanical
- Skills: Operation & troubleshooting of injectable machines, Autoclaves, Sterilizers
- Utility – Sr. Officer
- Experience: 4–6 years
- Qualification: B.E/B.Tech Electrical
- Skills: Boilers, Chillers, Compressors, Water Systems
- Blister/Cartonator – Jr. Operator
- Experience: 2–4 years
- Qualification: B.Sc / ITI / Diploma
- Skills: Blister packing, Cartonator operations, Troubleshooting
- Production (OSD – Granulation) – Jr. Operator
- Experience: 2–4 years
- Qualification: B.Sc / ITI / Diploma
- Skills: Granulation, Compression, Coating, Packing
- Production (Sterile – QMS/Autoclave) – Sr. Officer
- Experience: 4–6 years
- Qualification: B.Pharm / M.Pharm / M.Sc
- Skills: QMS documentation, Deviation handling, Autoclave operation, Sterility assurance
Eligibility / Qualifications
- Education: B.Pharm, M.Pharm, M.Sc, B.E/B.Tech (Mechanical/Electrical), ITI, Diploma
- Experience: 2–10 years depending on role
- Preferred Skills: Regulatory exposure (USFDA, MHRA, EU compliance), GMP knowledge, advanced equipment handling, QMS, HVAC systems.
Relevant courses: Pharmaceutical Chemistry, Industrial Pharmacy, Biotechnology, Microbiology, Electrical/Mechanical Engineering, Quality Assurance.
Location & Salary
- Work Location: USV Pvt. Ltd., Daman formulation site
- Walk-in Venue: USV Pvt. Ltd., Daman
- Date & Time: 14th September 2025 | 9:00 AM – 2:00 PM
- Salary: Competitive as per industry standards
Application Process
Interested candidates can attend the walk-in drive or send their CVs if unable to participate.
Walk-in: 14th September 2025 (Sunday)
Location: USV Pvt. Ltd., Daman (formulation site)
Email (if unable to attend): recruiterdamanusv@usv.in
Mention Department & Position in subject line
Carry: Salary slips, breakup, Aadhaar card, PAN card, photo, and educational certificates
Why Join USV Pharma?
- Work with a USFDA, MHRA, and EU-approved facility
- Opportunity to contribute to a company ranked #1 in Oral Anti-Diabetics
- Exposure to advanced equipment like Sejong, Glatt RMG, FBD, FBP, Cadmac, Gansons
- Strong growth opportunities in a compliance-driven environment
FAQs
1. Who can apply for these openings?
Candidates with qualifications in B.Pharm, M.Pharm, M.Sc, B.E/B.Tech, ITI, Diploma and relevant experience (2–10 years).
2. What is the walk-in date and location?
14th September 2025 at USV Pvt. Ltd., Daman.
3. Is regulatory exposure mandatory?
Yes, prior experience in USFDA/MHRA-regulated facilities is preferred.
4. Can I apply if I cannot attend the walk-in?
Yes, send your updated CV to recruiterdamanusv@usv.in.
5. What documents should I carry for the interview?
Salary slips, ID proof, educational certificates, and recent photo.
Job Summary Table
| Category | Details |
|---|---|
| Company | USV Private Limited |
| Vacancies | QA, QC, Production, Maintenance (multiple positions) |
| Required Education | B.Pharm, M.Pharm, M.Sc, B.E/B.Tech (Mech/Electrical), ITI, Diploma |
| Experience | 2–10 years depending on role |
| Location | Daman, India |
To apply for this job email your details to recruiterdamanusv@usv.in

