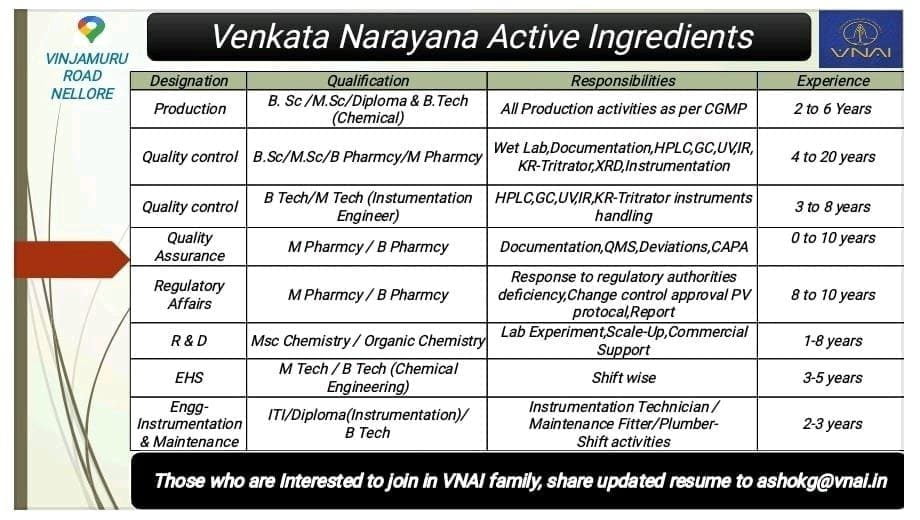
Multiple Pharma Jobs for fresher & Experience Candidates in Production, Regulatory affairs, QC, QA, EHS, RnD Departments 2022
- Production
- Quality Control
- Quality Control
- Quality Assurance
- Regulatory Affairs
- EHS
- Engineering Instrumentation & Maintenance
Venkata Narayana Active Ingredients Hiring fresher & Experience Candidates 2022
VINJAMURU ROAD NELLORE
Vacancies Information described Below.
Production
Qualification: BSc / MSc / Diploma & B Tech (Chemical)
Responsibilities: All Production activities as per CGMP
Experience: 2 to 6yrs
Quality Control
Qualification: BSc / MSc / B Pharmacy / M Pharmacy
Responsibilities: Wet Lab, Documentation HPLC, GC, UVIR, KR Tritrator, XRD,Instrumentation
Experience: 4 to 20 years
Quality Control
Qualification: B Tech/M Tech (Instumentation Engineer)
Responsibilities: HPLC, GC, UVIR, KR-Tritrator instruments handling
Experience: 3 to 8 years
Quality Assurance
Qualification: M Pharmacy / B Pharmacy
Responsibilities: Documentation, QMS, Deviations, CAPA
Experience:0 to 10 years
Regulatory Affairs
Qualification: M Pharmacy / B Pharmacy
Responsibilities: Response to regulatory authorities deficiency,Change control approval PV protocal, Report
Experience: 8 to 10 years.
Research & Development (R&D)
Qualification: Msc Chemistry / Organic Chemistry
Responsibilities: Lab Experiment, Scale-Up, Commercial Support
Experience: 1-8 years
EHS
Qualification: M Tech / B Tech (Chemical Engineering)
Responsibilities: Shift wise
Experience: 3 to 5 years
Engineering Instrumentation & Maintenance
Qualification: ITI / Diploma (Instrumentation) / B Tech
Responsibilities: Instrumentation Technician / Maintenance Fitter / Plumber Shift activities
Experience: 2-3 years
Those who are Interested to join in VNAI family, share updated resume to as****@vn**.in




