Shilpa Medicare Walk-In QC, IPM, RA (RoW/US/EU/CA)

- Join Shilpa Medicare: Hiring for Quality Control, Regulatory Affairs, and IPM Roles in Hyderabad
- Departments & Job Openings
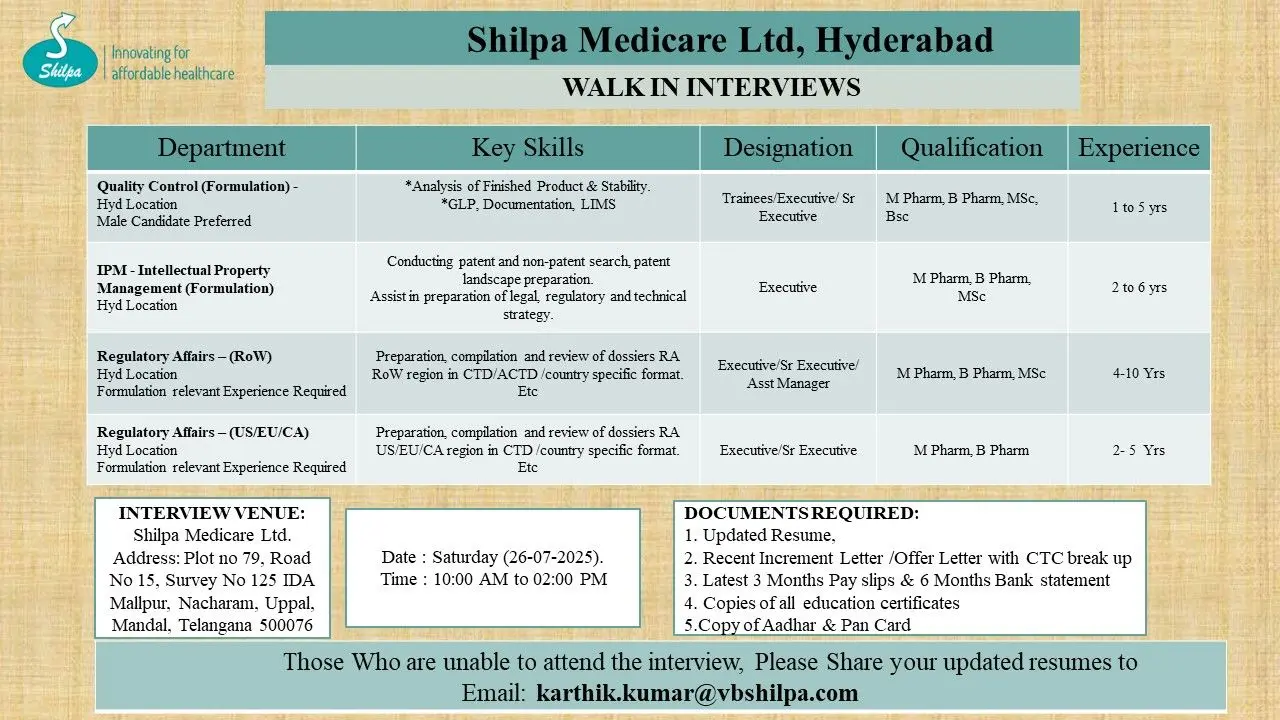
- Quality Control (Formulation) – Hyderabad
- Intellectual Property Management (IPM) – Hyderabad
- Regulatory Affairs (RoW) – Hyderabad
- Regulatory Affairs (US/EU/CA) – Hyderabad
- Documents to Carry
- Can’t Attend the Walk-In?
- Why Join Shilpa Medicare?
- Job Summary Table
Shilpa Medicare Hiring B.Pharm/M.Pharm/M.Sc Graduates for QC, Regulatory Affairs & IPM Roles in Hyderabad
Explore current pharma job openings at Shilpa Medicare Hyderabad for B.Pharm, M.Pharm, and M.Sc graduates in QC, Regulatory Affairs, and IPM with 1–10 years of experience.
Join Shilpa Medicare: Hiring for Quality Control, Regulatory Affairs, and IPM Roles in Hyderabad
👉 Never Miss a Pharma Job Again
💼 Join our LIVE WhatsApp Group & Get Instant Updates. 📢 Click below to join:
📱 Join Click HereShilpa Medicare Ltd, a leading name in innovative and affordable healthcare, is conducting walk-in interviews in Hyderabad for multiple pharma roles. If you’re a B.Pharm, M.Pharm, M.Sc, or B.Sc graduate with 1–10 years of relevant experience, this is your opportunity to grow in a reputed and regulatory-compliant pharmaceutical environment.
Walk-In Date: Saturday, 26 July 2025
Time: 10:00 AM to 2:00 PM
Location: Plot No. 79, Road No. 15, Survey No. 125, IDA Mallapur, Nacharam, Uppal Mandal, Telangana – 500076
Departments & Job Openings
Quality Control (Formulation) – Hyderabad
Designation: Trainee / Executive / Sr. Executive
Eligibility: B.Pharm / M.Pharm / M.Sc / B.Sc
Experience: 1–5 years
Key Skills:
- Finished Product & Stability Analysis
- Good Laboratory Practices (GLP)
- Documentation and LIMS systems
Note: Male candidates are preferred for this role.
Intellectual Property Management (IPM) – Hyderabad
Designation: Executive
Eligibility: M.Pharm / B.Pharm / M.Sc
Experience: 2–6 years
Key Responsibilities:
- Patent and non-patent search
- Preparing patent landscapes
- Support in legal and regulatory strategy formulation
Regulatory Affairs (RoW) – Hyderabad
Designation: Executive / Sr. Executive / Assistant Manager
Eligibility: M.Pharm / B.Pharm / M.Sc
Experience: 4–10 years
Job Profile:
- Compilation of dossiers for RoW markets in CTD/ACTD formats
- Experience in formulation-related submissions
Regulatory Affairs (US/EU/CA) – Hyderabad
Designation: Executive / Sr. Executive
Eligibility: M.Pharm / B.Pharm
Experience: 2–5 years
Job Role:
- Preparation and review of regulatory submissions for US, EU, and Canada
- Familiarity with CTD and country-specific formats
Documents to Carry
Candidates attending the walk-in must bring:
- Updated Resume
- Recent Increment/Offer Letter with CTC breakup
- Last 3 months’ payslips and 6 months’ bank statement
- Copies of all academic certificates
- PAN and Aadhar card copies
Can’t Attend the Walk-In?
No worries. You can email your updated resume to: karthik.kumar@vbshilpa.com
Why Join Shilpa Medicare?
- Exposure to regulated market standards (US/EU/CA)
- Opportunities in both formulation development and regulatory management
- Career growth with a leading pharma company committed to affordable healthcare
Job Summary Table
| Company Name | Shilpa Medicare Ltd |
|---|---|
| Vacancies | QC, IPM, RA (RoW/US/EU/CA) |
| Required Education | B.Pharm, M.Pharm, M.Sc, B.Sc |
| Experience Required | 1 to 10 Years |
| Job Location | Hyderabad, Telangana |