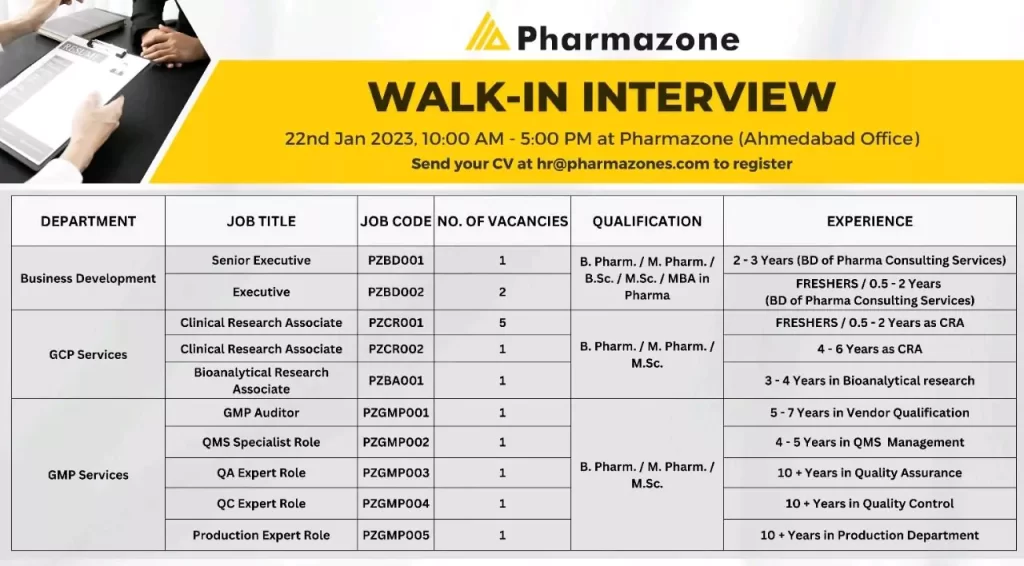
multiple job openings for fresher & experience

Multiple Vacancies At Pharmazone, Ahmedabad Location.
Pharmazone, hiring for multiple vacancies at the Ahmedabad location. (Office based only)
About pharmazone: highly focused GxP compliance and regulatory affairs service-oriented company established in 2009. We’re a company that supports the toughest GxP compliance and regulatory affairs challenges.
Open vacancies in Ahmedabad
GCP (Goods clinical practice)
Project Coordinator
- Experience: Fresher
- Qualification: B.Pharm/M.Pharm
- Prefer local candidates from Ahmedabad only
Project coordinator/Sr. Project coordinator – GCP Services (Clinical research associate)
- Experience: 2 to 5 years of BA/BE or Clinical trials studies
- Qualification: B.Pharm/M.Pharm
- CRA & CRC both can apply.
Bioanalytical monitor
- Experience: 2 to 5 years of Bioanalytical domain
- Qualification: B.Pharm/M.Pharm
GMP (Goods manufacturing practice)
- Position 1: GMP Auditor
- Experience: 3 to 7 years of experience of handling GMP audits or Vendor audits at API site
- Qualification: B.Pharm/M.Pharm
Regulatory Affairs
Manager – Regulatory Affairs EU market
- Experience: 8 to 15 years of experience in Regulatory Affairs for the Europe market
- Must-Have good communication and writing skills
- Must have Knowledge of updated regulatory guidance
- Must have Europe Market regulatory experience
- Must have experience in Formulation (FDF filing) of the Europe market.
- Compilation and review of MA/ANDA/NDA sections
- Handling of global regulatory submission and query response
- Compilation of dossier for Non-regulated markets (if any)
- RA team lead
- Managing day-to-day task of the RA team
Attending project-related technical discussions with clients
Executive/Sr. Executive – Regulatory Affairs India market
- Experience: 2 to 5 years of experience of India market compliance and registration
Pharmacovigilance
Pharmacovigilance Associate (Literature search only)
- Experience: 2 to 4 years of experience in literature search
Pharmacovigilance associate (ICSR – Drug cases)
- Experience: 2 to 4 years of experience in ICSR – Drug cases processing only
- Please note: processing of vaccine cases is not required for this position
Pharmacovigilance associates (Aggregate reports)
- Experience: 2 to 4 years of experience in PSURs, RMP, and/or PSMF drafting experience
Pharmacovigilance associate (MICC – Fresher)
Experience: Fresher
Prefer local candidates from Ahmedabad only
- Who will be responsible for working in Night shifts/rotational shift
- Will be responsible for handling medical information call center
- Excellent communication skill
- Qualification: B.Pharm/M.Pharm
Business Development
The hiring of a Business development professional for Regulatory affairs/ Pharmacovigilance services
Sr. Executive – Business Development (RA/PV services)
- Experience: 3 to 8 years
Market: Europe
Skill required: Experience of RA/PV/CRO services
Sr. Executive – Business Development (RA/PV services)
- Experience: 3 to 10 years
Market: USA
Skill required: Experience of RA/PV/CRO services
Executive – Business Development (RA/PV services)
- Experience: 2+ years
Market: Any
Skill required: Experience in RA/PV/CRO services
Application Process; Interested candidates can directly share CV on WhatsApp number: +918733982433
Regards,
Bhavesh Rami,
Sr. Executive – HR
Pharmazone, Ahmedabad.
+91 8733982433