Join the Exciting World of Regulatory Affairs at ZCL Chemicals Ltd

Join the Exciting World of Regulatory Affairs at ZCL Chemicals Ltd – Apply Now
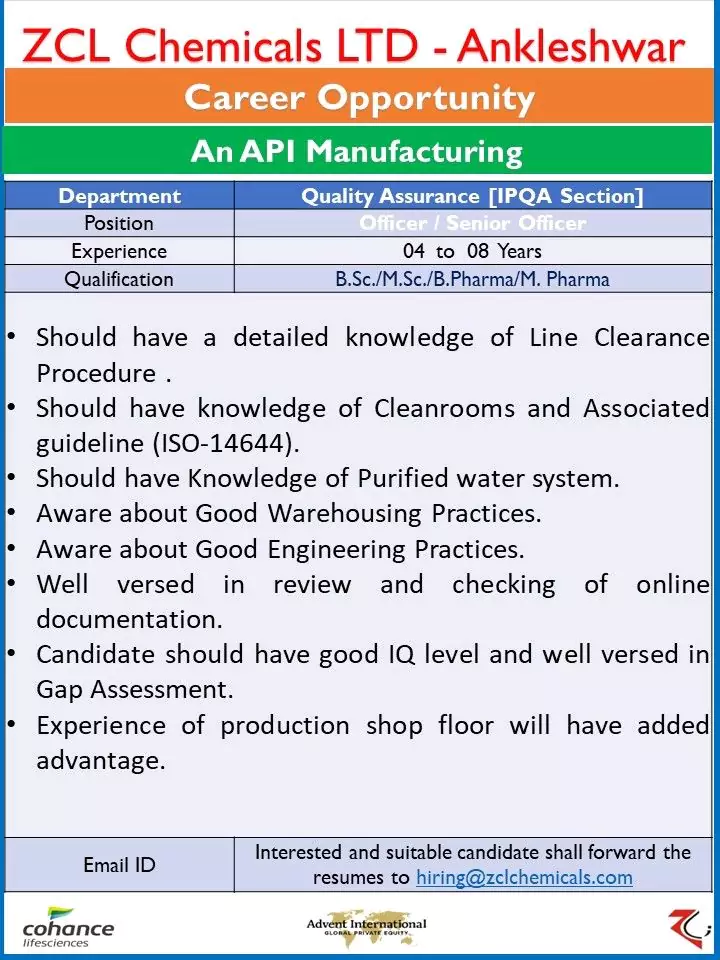
ZCL Chemicals Ltd is a leading player in the API Pharma manufacturing industry, with a strong focus on quality and compliance. As part of its growth plans, the company is seeking experienced professionals for its Regulatory Affairs department. The role offers a challenging and rewarding career opportunity for individuals with a strong background in regulatory compliance and API exposure.
ZCL Chemicals Ltd Job Vacancy For Regulatory Affairs Department
Regulatory Affairs (API Exposure)
- Designation:- Senior Officer / Executive
- Qualifications: M.Sc. (Organic Chemistry) / M. Pharma (RA)
- Experience:- 3 to 6 years of Experience in core RA in API Pharma manufacturing is preferable.
Work Profile:-
- Technology Transfer document review for adequacy with respect to the regulatory requirements.
- Compilation & review of Drug Master File for regulatory filings. Regulatory communication with the marketing department.
- Filing of annual reports and amendments.
- Preparation of response for DMF deficiencies received from the agency as well customers.
- Evaluation of change with respect to regulatory impact and informed customers through marketing.
- Coordination with other departments for regulatory requirements including deficiency response.
- To ensure meeting a scheduled date for DMF filing as per the planner discussed and agreed upon.
- Coordination with another department for customer questionnaire requirements.
- CGMP and GLP-related compliance.
- Well-versed with Pharma Reddy Software.
APPLICATION PROCESS; Interested candidates can drop their updated CV to Hi****@zc**********.com
To learn more about ZCL Chemicals and apply for the job opening, visit the career page at https://www.zclchemicals.com/careers/