Eris Therapeutics Walk-In Interview | QC, QA, Packing, Engineering, F&D Roles

- Eris Therapeutics Walk-In Interview for Exciting Career Opportunities
- Walk-In Interview Details
- Available Positions and Requirements
- How to Apply
- Tips for Candidates
Eris Therapeutics Walk-In Interview for Exciting Career Opportunities
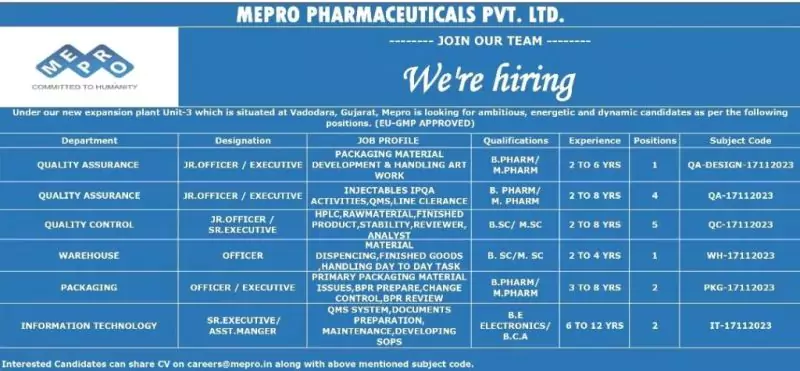
Eris Therapeutics invites talented professionals to its walk-in interview for various positions in Quality Control (QC), Quality Assurance (QA), Packing, Engineering, and Formulation and Development (F&D). This is your chance to join a leading pharmaceutical organization dedicated to innovation and excellence.
Walk-In Interview Details
Date: Sunday, 8th December 2024
Time: 10:00 AM to 4:00 PM
Venue:
Corporate Office, Plot No. 142/2, Ramdas Road, Off SBR,
Near Swati Bunglows, Bodakdev, Ahmedabad – 380054
📄 Get Your Dream Job with a Professional Resume!
💼 Struggling to Get Noticed by Recruiters? 📄 Let Professionally Crafted Resumes Boost Your Career! 🚀
📱 Get More DetailsBring along your updated resume, educational certificates, experience letters, last three months’ salary slips, and recent photographs.
Available Positions and Requirements
1. Quality Control (QC)
Designation: Officer/Sr. Officer
Qualification: M.Sc. (Chemistry), B.Pharm
Experience: 2–7 years
Key Skills:
- Analytical testing of finished goods, tablets, capsules, injectables, and external preparations.
- Proficient in operating HPLC, GC, Dissolution, and other instruments.
2. Quality Assurance (QA)
Designation: Officer/Sr. Officer
Qualification: B.Pharm, M.Pharm
Experience: 2–5 years
Key Responsibilities:
- Managing QA processes, documentation, and compliance with regulatory standards.
- Knowledge of in-process quality checks for oral solid dosage forms and injectables.
3. Packing
Designation: Executive/Sr. Executive
Qualification: B.Pharm, M.Pharm
Experience: 5–7 years
Key Responsibilities:
- Oversee packing operations for injectables and external preparations.
- Ensure adherence to GMP guidelines and efficient manpower management.
4. Engineering
Designation: Trainee/Assistant Manager
Qualification: Diploma/Bachelor’s in Mechanical or Electrical Engineering
Experience: 1–2 years
Key Skills:
- Machine troubleshooting, operation, and maintenance.
- Hands-on experience with capsule and OSD manufacturing equipment.
5. Formulation and Development (F&D)
Designation: Sr. Executive/Assistant Manager
Qualification: M.Pharm
Experience: 3–7 years
Key Responsibilities:
- Perform analytical development, validation, and stability activities.
- Handle regulatory compliance for pharmaceutical drug products.

How to Apply
- Walk-In: Attend the walk-in interview at the given venue on 8th December 2024.
- Email: Send your resume to harish.deshpande@eristherapeutics.com or recruitment@eristherapeutics.com.
- WhatsApp: Share your details at 9775417204 or 8447888966.
Tips for Candidates
- Arrive on time for the interview with all required documents.
- Highlight your skills and experience relevant to the position you are applying for.
- Be prepared to discuss your contributions in previous roles.