Concord Biotech Walk-in QC Reviewer, HPLC Analyst, ADL Executive

- Concord Biotech Limited: Walk-In Interview for QC, HPLC Analyst, and ADL Roles
- Job Interview Details:
- Available Job Positions at Concord Biotech
- Reviewer – Quality Department
- HPLC Analyst – QC Department
- Sr. Executive / Executive – ADL Department
- Why Join Concord Biotech?
- How to Apply?
- Summary Table for SEO
Hiring HPLC Analysts, QC Reviewers & ADL Executives | BSc, MSc, B.Pharm Jobs in Ahmedabad (5+ Openings)
Explore job opportunities at Concord Biotech for BSc, MSc, and B.Pharm candidates in QC, ADL & Review roles at Ahmedabad, Gujarat. Interview on 18–19 July 2025.
Concord Biotech Limited: Walk-In Interview for QC, HPLC Analyst, and ADL Roles
Concord Biotech, a leading name in biotech manufacturing, is conducting a walk-in interview for multiple positions across Quality Control (QC), Analytical Development Laboratory (ADL), and Review departments at their Unit-II facility in Valthera, Dholka, Ahmedabad, Gujarat.
👉 Never Miss a Pharma Job Again
💼 Join our LIVE WhatsApp Group & Get Instant Updates. 📢 Click below to join:
📱 Join Click HereThis opportunity is ideal for professionals with qualifications in B.Sc, M.Sc (Analytical Chemistry), or B.Pharm and hands-on experience in pharmaceutical quality operations.
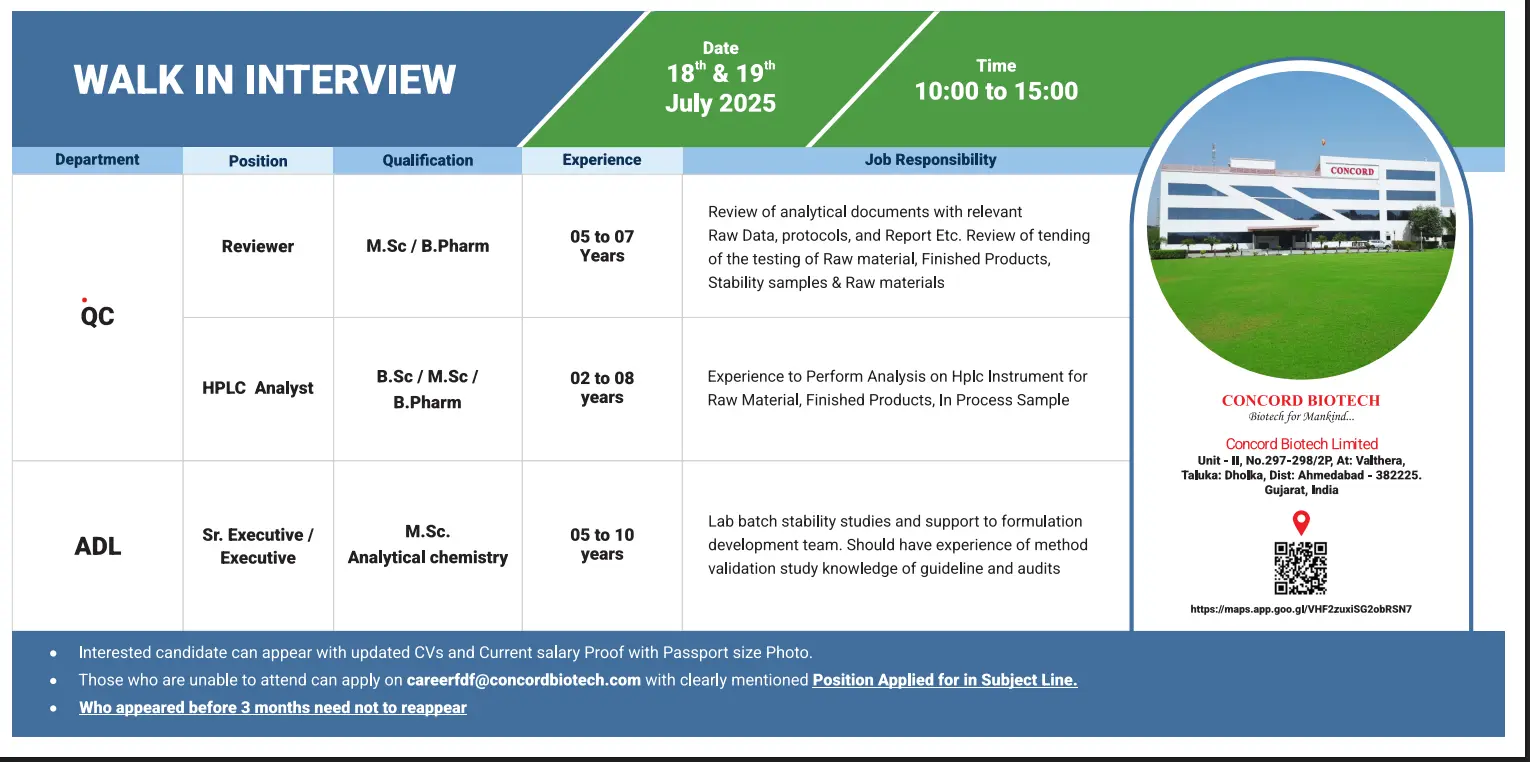
Job Interview Details:
Walk-in Dates: 18th & 19th July 2025
Timing: 10:00 AM to 03:00 PM
Location: Concord Biotech Ltd., Unit-II, No.297-298/2P, At: Valthera, Taluka: Dholka, Dist: Ahmedabad – 382225, Gujarat, India
📌 Google Maps Link
Available Job Positions at Concord Biotech
Reviewer – Quality Department
Qualification: M.Sc / B.Pharm
Experience: 5 to 7 Years
Key Responsibilities:
- Review of analytical documentation, protocols, and reports
- Audit readiness for raw material, finished products, and stability data
HPLC Analyst – QC Department
Qualification: B.Sc / M.Sc / B.Pharm
Experience: 2 to 8 Years
Key Responsibilities:
- Conduct HPLC analysis of RM, FP, and in-process samples
- Maintain accurate instrumentation records as per cGMP guidelines
Sr. Executive / Executive – ADL Department
Qualification: M.Sc Analytical Chemistry
Experience: 5 to 10 Years
Key Responsibilities:
- Execute lab batch stability studies
- Support formulation development
- Perform method validation and ensure compliance with regulatory standards
Why Join Concord Biotech?
- Exposure to a cGMP and regulatory-compliant environment
- Opportunities in advanced analytical research and QC operations
- Career growth within a globally reputed biotech firm
How to Apply?
Walk-in with: Updated CV, current salary proof, and passport-size photo
Candidates who appeared within the last 3 months need not reapply
Can’t attend? Send your resume to: careerfdf@concordbiotech.com with subject line “Position Applied For”
Summary Table for SEO
| Company Name | Current Vacancies | Required Education | Experience Required |
|---|---|---|---|
| Concord Biotech Ltd. | QC Reviewer, HPLC Analyst, ADL Executive | B.Sc, M.Sc (Analytical), B.Pharm | 2–10 Years |