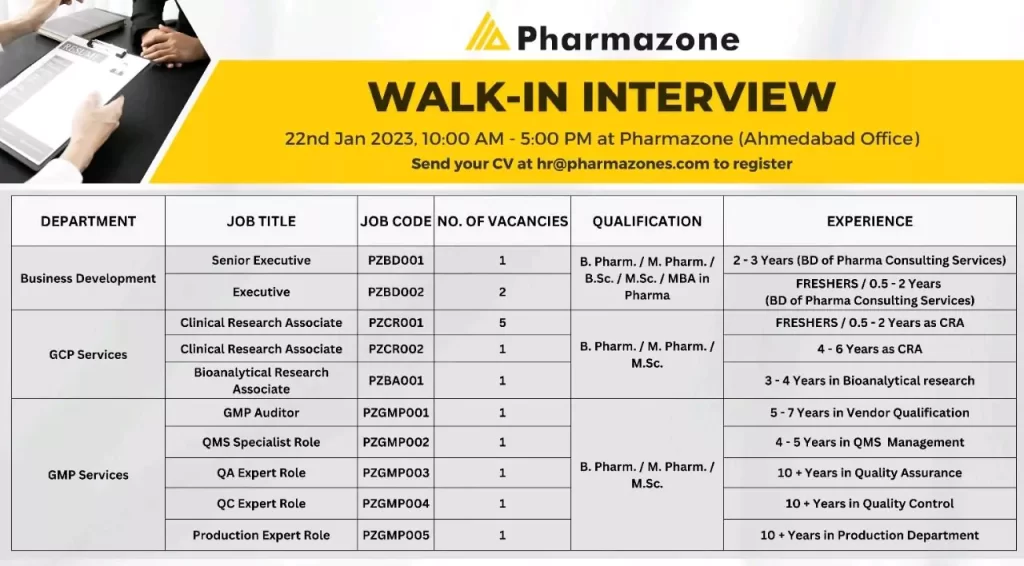
Accenture Pharmacovigilance fresher jobs; Bangalore
- Accenture Pharmacovigilance fresher jobs
- Introduction
- About Accenture
- Job Overview
- Responsibilities and Authorities
- Required Skills and Qualifications
- Educational and Experience Requirements
- Training and Development Opportunities
- How to Apply
- APPLY HERE
Accenture Pharmacovigilance fresher jobs
Are you a fresh graduate looking for exciting career opportunities in pharmacovigilance? Accenture, a global leader in professional services, is actively hiring freshers for Pharmacovigilance roles. As a Junior Drug Safety Associate at Accenture, you will be part of a dynamic team responsible for detecting, assessing, and preventing adverse effects and medicine-related issues. With a strong emphasis on teamwork, adaptability, and commitment to quality, Accenture offers a supportive environment for your professional growth. If you hold a degree in Health Sciences or Life Sciences and have a passion for ensuring patient safety, don’t miss the chance to kick-start your career with Accenture’s Pharmacovigilance fresher jobs. Apply today for this exciting opportunity.
Introduction
In this article, we will provide a comprehensive job description for the position of Junior Drug Safety Associate in the field of Pharmacovigilance. Pharmacovigilance plays a crucial role in ensuring drug safety and monitoring adverse effects. As a Junior Drug Safety Associate, you will be part of a dynamic team responsible for various aspects of pharmacovigilance, including adverse event detection, assessment, understanding, prevention, and reporting. This article will outline the qualifications, responsibilities, and required skills for this position.
About Accenture
Accenture is a leading global professional services company that specializes in digital, cloud, and security solutions. With extensive experience across more than 40 industries, Accenture offers a wide range of services in strategy and consulting, technology, operations, and more. Their Life Sciences R&D vertical focuses on supporting the entire life sciences enterprise, including pharmacovigilance and patient services solutions. Accenture aims to leverage technology and human ingenuity to drive positive change and create value for clients, employees, shareholders, partners, and communities.
👉 Never Miss a Pharma Job Again
💼 Join our LIVE WhatsApp Group & Get Instant Updates. 📢 Click below to join:
📱 Join Click HereTo learn more about Accenture and their services, please visit www.accenture.com.
Job Overview
Designation: Junior Drug Safety Associate
Job Location: Bengaluru
As a Junior Drug Safety Associate, you will be part of Accenture’s Pharmacovigilance team. Your role will involve working on various activities related to pharmacovigilance, such as adverse event case processing, assessment, and reporting. You will be responsible for ensuring compliance with Good Pharmacovigilance Practice (GVP) guidelines, client SOPs, and global regulatory requirements. This position requires a detail-oriented individual with excellent analytical, communication, and medical writing skills.
Responsibilities and Authorities
As a Junior Drug Safety Associate, your responsibilities and authorities will include:
- Prioritizing case processing activities for Individual Case Safety Reports (ICSRs) based on project guidelines, regulatory due dates, and TAT SLAs and KPIs.
- Processing and evaluating Individual Case Safety Reports accurately and consistently from source documents within defined timelines.
- Ensuring correct coding of medical history, drugs, and adverse event terms.
- Assessing adverse event reports for seriousness, causality, and expectedness according to applicable labeling, consulting the Medical Reviewer when necessary.
- Alerting the Medical Reviewer of potential safety issues and assisting in monitoring the safety profile of products.
- Gathering additional information, if required, to clarify or follow up on cases.
- Archiving all communications and clarifications related to cases in the Global Safety Database.
- Initiating and handling case deletions or nullifications when appropriate.
- Participating in audits or inspections.
- Being open to training and moving across roles based on business requirements.
- Providing training and mentoring to new associates.
- Authoring process documents as needed.
Required Skills and Qualifications
To excel in the role of Junior Drug Safety Associate, the following skills and qualifications are necessary:
- Ability to work well in a team and collaborate effectively.
- Adaptability and flexibility in a dynamic work environment.
- Agility for quick learning and staying updated with industry developments.
- Commitment to quality and attention to detail.
- Educational background in health sciences or life sciences, such as a Bachelor’s degree in Pharmacy or a related field.
- Minimum of 1 year of experience in pharmacovigilance, pharmaceuticals, or clinical research preferred.
- Knowledge of medical terminology, including familiarity with dictionaries like MedDRA and WHO-Drug.
- Strong medical writing skills.
- Excellent communication skills, both written and verbal.
- Analytical ability to evaluate and process adverse events accurately.
- Proficiency in the English language.
Educational and Experience Requirements
To be eligible for the role of Junior Drug Safety Associate, the following educational and experience requirements should be met:
- Minimum of a Bachelor’s degree in Health Sciences, or a Bachelor’s Degree in Life Sciences with relevant pharmacovigilance experience.
- Preferably, a minimum of 1 year of experience in pharmaceuticals or clinical research.
- Strong knowledge of medical terminology, including MedDRA and WHO-Drug.
- Proficient medical writing skills.
- Excellent communication skills, both written and verbal.
- Analytical ability to evaluate and process adverse events accurately.
- Strong proficiency in the English language.
Training and Development Opportunities
Accenture recognizes the importance of continuous learning and professional development. As a Junior Drug Safety Associate, you can expect the following training and development opportunities:
- Comprehensive onboarding and orientation programs to familiarize you with Accenture’s pharmacovigilance processes, systems, and best practices.
- Ongoing training sessions to enhance your knowledge of pharmacovigilance guidelines, regulatory requirements, and industry trends.
- Access to internal and external training resources, workshops, and webinars to strengthen your skills and expertise.
- Mentoring and guidance from experienced professionals in the pharmacovigilance field.
- Opportunities to attend conferences, seminars, and industry events to stay updated with the latest advancements in pharmacovigilance.
How to Apply
APPLY HERE
To apply for the position of Junior Drug Safety Associate at Accenture, please follow these steps:
- Click on the job posting to access the detailed description and requirements.
- Review the qualifications and responsibilities to ensure a good fit.
- Click on the “Apply Now” button to start the application process.
- Fill in the required information, including your personal details, educational background, and work experience.
- Upload your updated resume and any other requested documents.
- Complete any additional assessments or questionnaires, if applicable.
- Review your application to ensure accuracy and completeness.
- Submit your application.