Emcure Pharmaceuticals Hiring QC (Microbiology) and QA Roles
- About the Company
- Company Vacancies List
- Job Description
- About the Department & Responsibilities
- How to Apply
Emcure Pharmaceuticals Job Vacancies: Empowering Careers in Quality Control and Assurance
About the Company
Welcome to Emcure Pharmaceuticals, a globally recognized pharmaceutical company committed to delivering high-quality healthcare solutions. With a focus on innovation, Emcure has been at the forefront of providing life-saving medications and is dedicated to attracting top talent to further its mission.
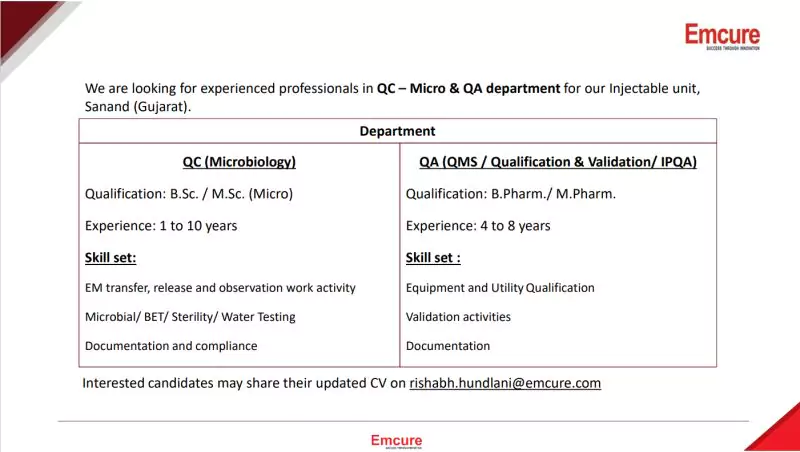
Company Vacancies List
Position Titles:
- QC (Microbiology)
- Qualification: B.Sc. / M.Sc. (Micro)
- Experience: 1 to 10 years
- Skill Set: Microbial/BET/Sterility/Water Testing, EM transfer, release, and observation work activity
- QA (QMS/Qualification & Validation/IPQA)
- Qualification: B.Pharm./M.Pharm.
- Experience: 4 to 8 years
- Skill Set: Validation activities, Documentation and compliance, Equipment and Utility Qualification

Job Description
QC (Microbiology)
Join our Microbiology team and contribute to the quality assurance of our Injectable unit in Sanand, Gujarat. Engage in activities such as microbial, BET, sterility, and water testing. Your expertise in EM transfer, release, and observation work will play a crucial role in maintaining our high standards.
QA (QMS/Qualification & Validation/IPQA)
📄 Get Your Dream Job with a Professional Resume!
💼 Struggling to Get Noticed by Recruiters? 📄 Let Professionally Crafted Resumes Boost Your Career! 🚀
📱 Get More DetailsAs part of the Quality Assurance team, you’ll be responsible for validation activities, ensuring documentation compliance, and conducting equipment and utility qualification. Make a significant impact on the processes that define our quality standards.
About the Department & Responsibilities
QC (Microbiology)
- Department: Quality Control
- Responsibilities:
- Microbial, BET, Sterility, and Water Testing
- EM Transfer, Release, and Observation Work Activity
QA (QMS/Qualification & Validation/IPQA)
- Department: Quality Assurance
- Responsibilities:
- Validation Activities
- Documentation and Compliance
- Equipment and Utility Qualification
How to Apply
Are you ready to contribute to Emcure’s legacy of excellence? Interested candidates may share their updated CVs at rishabh.hundlani@emcure.com.








