QA Executive Vacancy at Alembic Limited – Vadodara
QA Executive Vacancy at Alembic Limited – Vadodara: Quality Assurance Job Opportunity
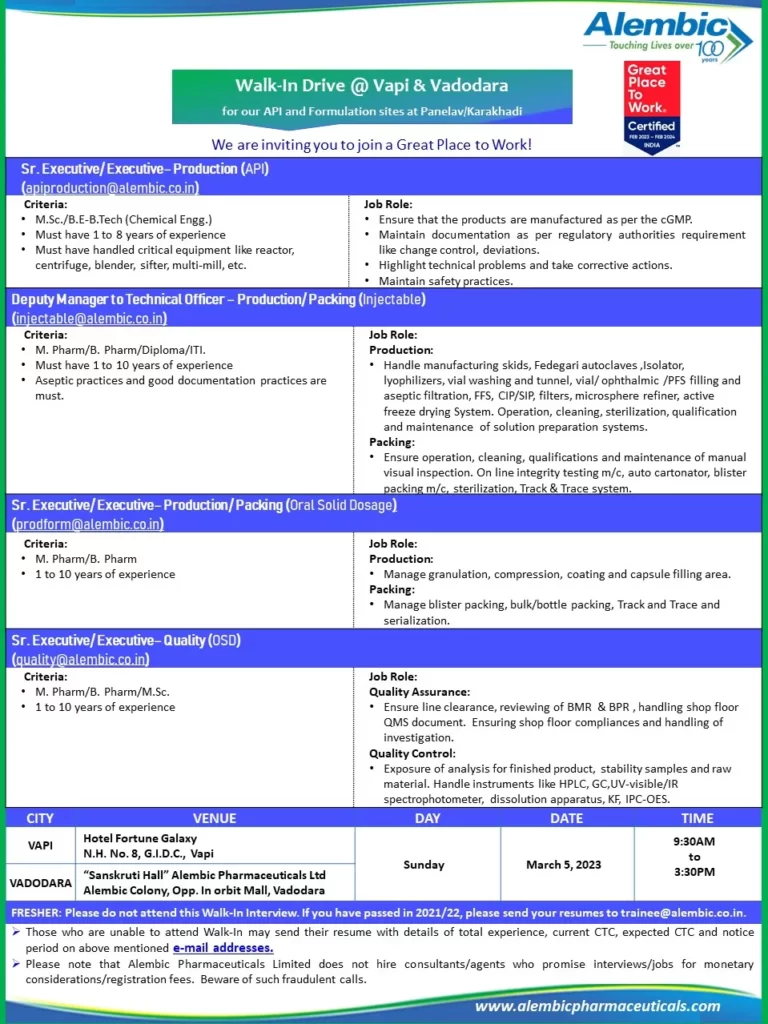
Alembic Limited, a renowned pharmaceutical company located in Vadodara, is on the lookout for a skilled and experienced QA Executive to join their API Plant in Gorwa. If you are a quality-focused professional with a background in chemistry and a deep understanding of cGMP and regulatory requirements, this could be the perfect opportunity for you to advance your career.
Alembic Limited QA Job Vacancies
Position Title: QA Executive
👉 Never Miss a Pharma Job Again
💼 Join our LIVE WhatsApp Group & Get Instant Updates. 📢 Click below to join:
📱 Join Click HereCompany Name: Alembic Limited
Company Address:
Detailed Job Description:
Role: QA Executive
Industry Type: Pharmaceutical
Department: Quality Assurance
Employment Type: Full-Time
Role Category: Quality Management
Education:
- UG: BSc in Chemistry
- PG: MSc in Chemistry
Job Description of QA Executive
As a QA Executive at Alembic Limited’s API Plant in Gorwa, your role will be instrumental in ensuring the quality and compliance of pharmaceutical products. You will be responsible for a range of tasks, including:
Quality Management System:
- Demonstrate a strong understanding of cGMP (Current Good Manufacturing Practices) and stay up to date with current regulatory requirements.
- Handle various aspects of the Quality Management System, including Change Control, Deviation, Incident Management, GDP (Good Documentation Practices), QRM (Quality Risk Management), APQR (Annual Product Quality Review), Trend Analysis, Market Complaint Handling, and the preparation of Standard Operating Procedures (SOPs).
Qualification and Experience:
- A bachelor’s degree (BSc) or master’s degree (MSc) in Chemistry is required for this position.
- The ideal candidate should have 4 to 8 years of relevant experience in a similar role.
Key Skills:
To excel in this role, the following key skills are essential:
- In-depth knowledge of cGMP and its application in a pharmaceutical setting.
- Proficiency in the Quality Management System, with the ability to handle Change Control, Deviation, Incident Management, GDP, QRM, APQR, Trend Analysis, Market Complaint Handling, and SOP preparation.
- Strong analytical and problem-solving skills to ensure the highest levels of quality and compliance.
How to Apply
If you meet the qualifications and have the experience required for this role, we encourage you to apply. Please submit your updated CV to rutvi.gandhi@alembic.co.in.