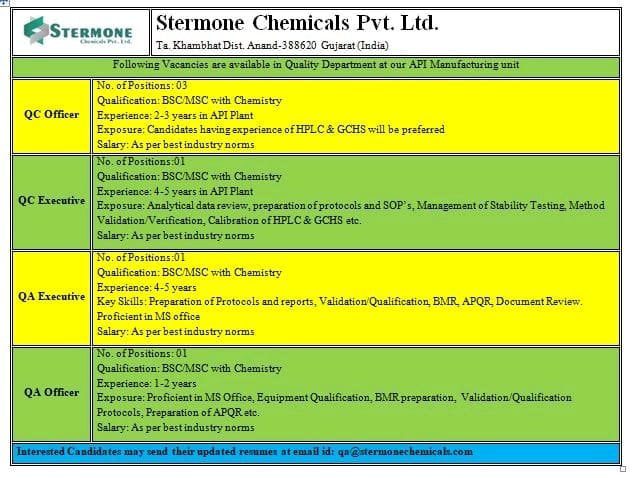
Quality Assurance Executive & Officers Level Job openings for Bsc, Msc Candidates at stermone Chemicals

Stermone Chemicals Pvt. Ltd Hiring Notification for Bsc, Msc Candidates for Quality assurance, Quality Control Departments.
Stermone Chemicals Pvt. Ltd. is an API Manufacturing Company mainly deals with Corticosteroid,based in Khambhat Dist. Anand, Gujarat.
It is a fast growing Pharma Compnay having GMP Certification from Gujarat FDCA and going to apply for WHO GMP Certification soon.
Following Vacancies are available in Quality Department at our API Manufacturing unit
Quality Assurance Executive
No. of Positions: 01
Qualification: BSC / MSC with Chemistry
Experience: 4-5 years
Key Skills:
Preparation of Protocols and reports. Validation / Qualification, BMR. APQR. Document Review.
Proficient in MS office
Salary: As per best industry norms
Quality Assurance Officer
No. of Positions: 01
Qualification: BSC / MSC with Chemistry
Experience: 1-2 years
Exposure:
Proficient in MS Office, Equipment Qualification, BMR preparation, Validation / Qualification, Protocols, Preparation of APQR etc.
Salary: As per best industry norms
Application Process : Interested Candidates may send their updated resumes at email id: [email protected]
Location : Ta. Khambhat Dist. Anand-388620 Gujarat (India).