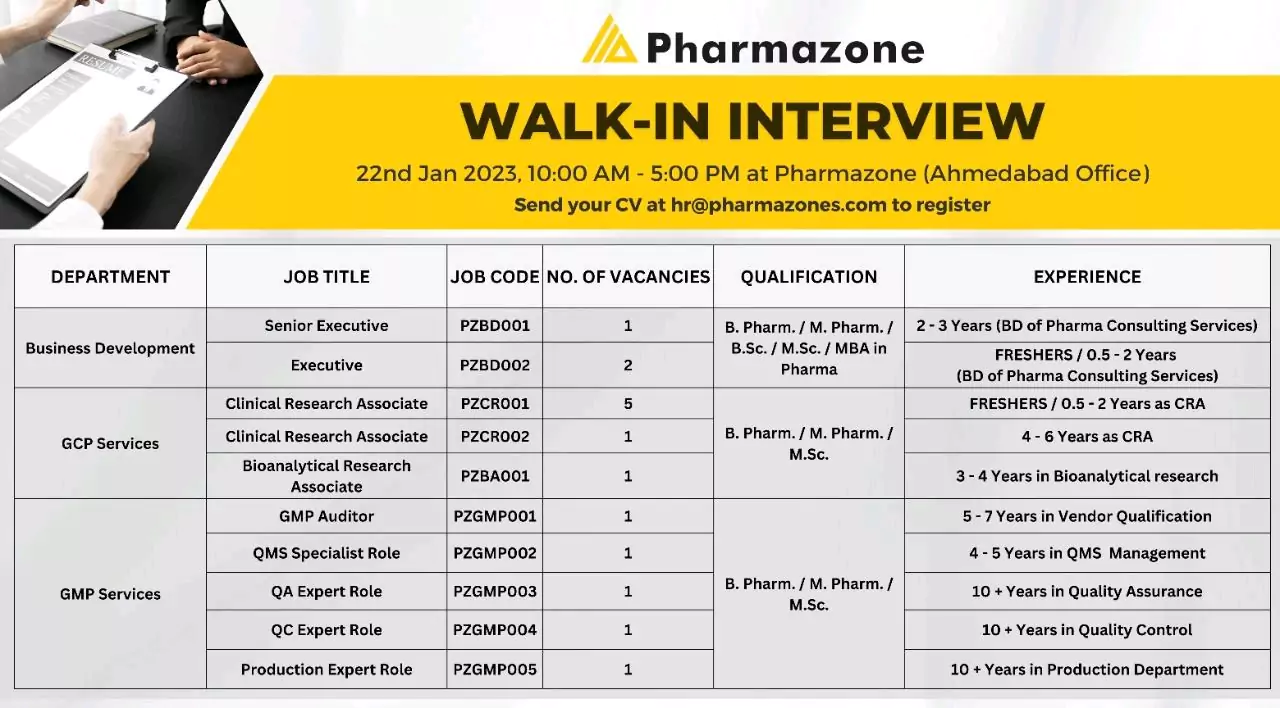
Walk in interview for clinical research, Bioanalytical, QMS, QA, QC – GCP, GMP Services
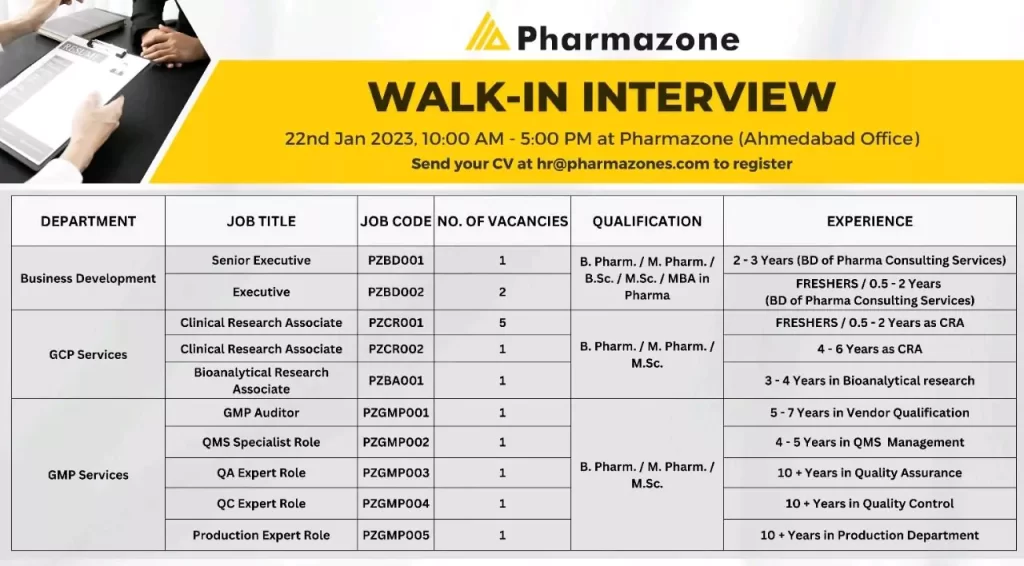
Pharmazone WALK-IN INTERVIEW 22nd Jan 2023, 10:00 AM – 5:00 PM at Pharmazone (Ahmedabad Office) for multiple positions
Business Development; The Business Development (BD) department in the pharmaceutical industry plays a crucial role in the growth and expansion of a company. The main responsibilities of the BD department include identifying and evaluating new business opportunities, such as licensing agreements, mergers and acquisitions, and partnerships. The department also plays a key role in the negotiation and execution of these deals.
Additionally, the BD department is responsible for identifying and evaluating potential new markets for the company’s products, as well as new products or technologies that could be developed or acquired to complement the company’s existing portfolio. They also work closely with other departments within the company, such as R&D, marketing, and operations, to ensure that any new business opportunities align with the company’s overall strategy and goals.
In summary, the Business Development department in the pharmaceutical industry is a strategic function that helps companies to grow and expand their business by identifying and developing new opportunities, partnerships, and collaborations.
Senior Executive PZBD001
Executive
Required Education: B Pharm / M Pharm / BSc /MSc / MBA in Pharma
Required Experience: 2-3 Years (BD of Pharma Consulting Services)
For Executive: FRESHERS / 0.5-2 Years (BD of Pharma Consulting Services)
Clinical Research Associate – PZCR001
Clinical Research Associate – PZCRO02
Bioanalytical Research Associate – PZBA001
Required Education: B. Pharm. / M. Pharm. / M.Sc.
Experience: FRESHERS / 0.5- 06Years as CRA or Bioanalytical Research Associate – 3-4 Years in Bioanalytical research
A Clinical Research Associate (CRA) is a professional who works in the pharmaceutical or biotechnology industry, typically at a Contract Research Organization (CRO), to manage and monitor clinical trials. The CRA plays a critical role in the conduct of clinical trials by ensuring that the trials are conducted according to Good Clinical Practice (GCP) guidelines, regulatory requirements, and the study protocol.
The specific responsibilities of a CRA may include:
- Reviewing and monitoring study progress and data
- Ensuring that study sites are properly trained and have the necessary equipment and supplies
- Conducting on-site monitoring visits to ensure that study conduct is in compliance with GCP guidelines
- Reviewing and tracking adverse events and serious adverse events
- Assisting with the preparation of study-related documents, such as informed consent forms, case report forms, and study protocols
- Communicating with the study sponsor and other members of the study team to ensure that the study is progressing as planned
- Helping to close out the study by ensuring that all study-related documents are complete and accurate
GMP Services
Available positions;
- GMP Auditor – PZGMP001;A Good Manufacturing Practices (GMP) auditor is a professional who is responsible for evaluating and assessing the compliance of a company’s manufacturing operations with GMP regulations. GMP is a set of guidelines and regulations established by regulatory agencies, such as the FDA, to ensure that pharmaceutical products are manufactured consistently and to a high quality standard.
The specific responsibilities of a GMP auditor may include:
- Reviewing and assessing a company’s GMP compliance program
- Conducting on-site inspections of manufacturing facilities to evaluate compliance with GMP regulations
- Reviewing and assessing documentation, such as Standard Operating Procedures (SOPs) and batch records
- Identifying and reporting any GMP non-compliance issues or deviations from established procedures
- Communicating with company management to ensure that identified non-compliance issues are addressed in a timely manner
- Participating in the development of GMP training programs for company employees
- Keeping abreast of changes in GMP regulations and guidelines and communicating these changes to company management.
- QMS Specialist Role – PZGMP002; A Quality Management System (QMS) specialist is a professional who is responsible for implementing, maintaining and continuously improving a company’s QMS.
- QA Expert Role – PZGMP003; A Quality Assurance (QA) expert in a Contract Research Organization (CRO) plays a crucial role in ensuring that clinical trials are conducted in compliance with Good Clinical Practice (GCP) guidelines and regulatory requirements
- QC Expert Role – PZGMP004; A Quality Control (QC) expert is a professional who is responsible for ensuring the quality of products manufactured by a company
- Production Expert Role – PZGMP005; A Production expert in a Contract Research Organization (CRO) plays a crucial role in the development, manufacture, and supply of investigational medicinal products (IMPs) for clinical trials
Required Education: B. Pharm. / M. Pharm. / M.Sc.
Required Experience :
- 5-7 Years in Vendor Qualification
- 4-5 Years in QMS Management
- 10+ Years in Quality Assurance
- 10+ Years in Quality Control
- 10+ Years in the Production Department
Registration Process: Send your CV to [email protected] to register / You can also do Whatsapp by sending an updated Cv to +918733982433 for registration.