Vital Pharma multiple Pharma Job vacancies in Hyderabad, QA, QC, AR&D, FR&D, Production & Store Departments
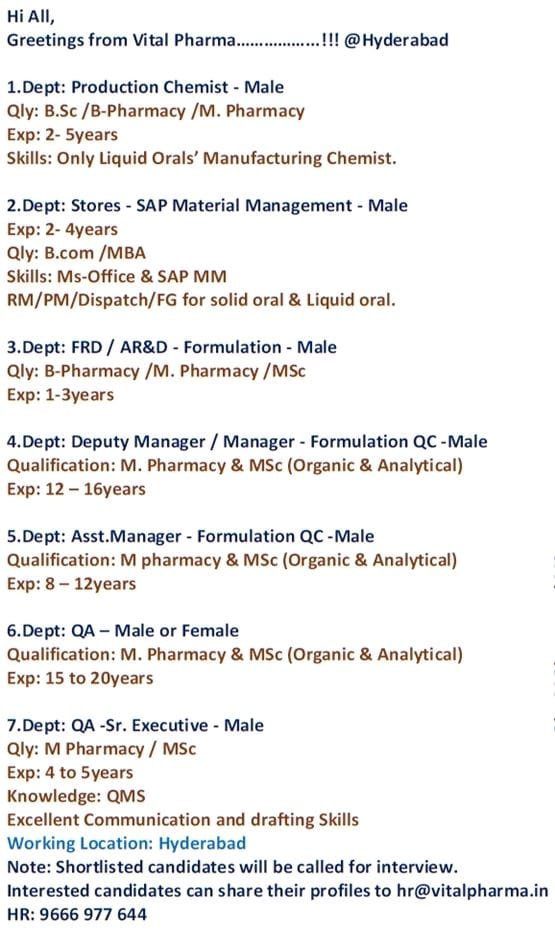
- Production Chemist – Male
- Stores – SAP Material Management – Male
- FRD / AR&D – Formulation – Male
- Deputy Manager / Manager – Formulation QC -Male
- Asst.Manager – Formulation QC -Male
- QA – Male or Female
- Quality Assurance (QA)
Vital Pharma Recruitment Notification for Multiple Departments at Hyderabad
Vacancies Details :
Production Chemist – Male
- Qualification: BSc / B Pharmacy /M Pharmacy
- EXPERIENCE: 2-5years
- Skills: Only Liquid Orals’ Manufacturing Chemist.
Stores – SAP Material Management – Male
- EXPERIENCE: 2- 4years
- Qualification: Bcom / MBA
- Skills: Ms-Office & SAP MM RM/PM/Dispatch/FG for solid oral & Liquid oral.
FRD / AR&D – Formulation – Male
- Qualification: B Pharmacy / M Pharmacy / MSc
- EXPERIENCE: 1-3years
Deputy Manager / Manager – Formulation QC -Male
- Qualification: M Pharmacy & MSc (Organic & Analytical)
- EXPERIENCE: 12-16years
Asst.Manager – Formulation QC -Male
- Qualification: M pharmacy & MSc (Organic & Analytical)
- EXPERIENCE: 8 – 12years
QA – Male or Female
- Qualification: M Pharmacy & MSc (Organic & Analytical)
- EXPERIENCE: 15 to 20years
Quality Assurance (QA)
- Designation : Sr. Executive – Male
- Qualification: M Pharmacy / MSc
- EXPERIENCE: 4 to 5years
- Knowledge: QMS
Excellent Communication and drafting Skills
Working Location: Hyderabad
Note: Shortlisted candidates will be called for interview.
Interested candidates can share their profiles to [email protected]
HR: 9666 977 644




