TEYRO LABS hiring FDD- Non-Sterile, quality assurance multiple job openings in tamilnadu
TEYRO LABS PRIVATE LIMITED Multiple Pharma Job openings in Tamilnadu
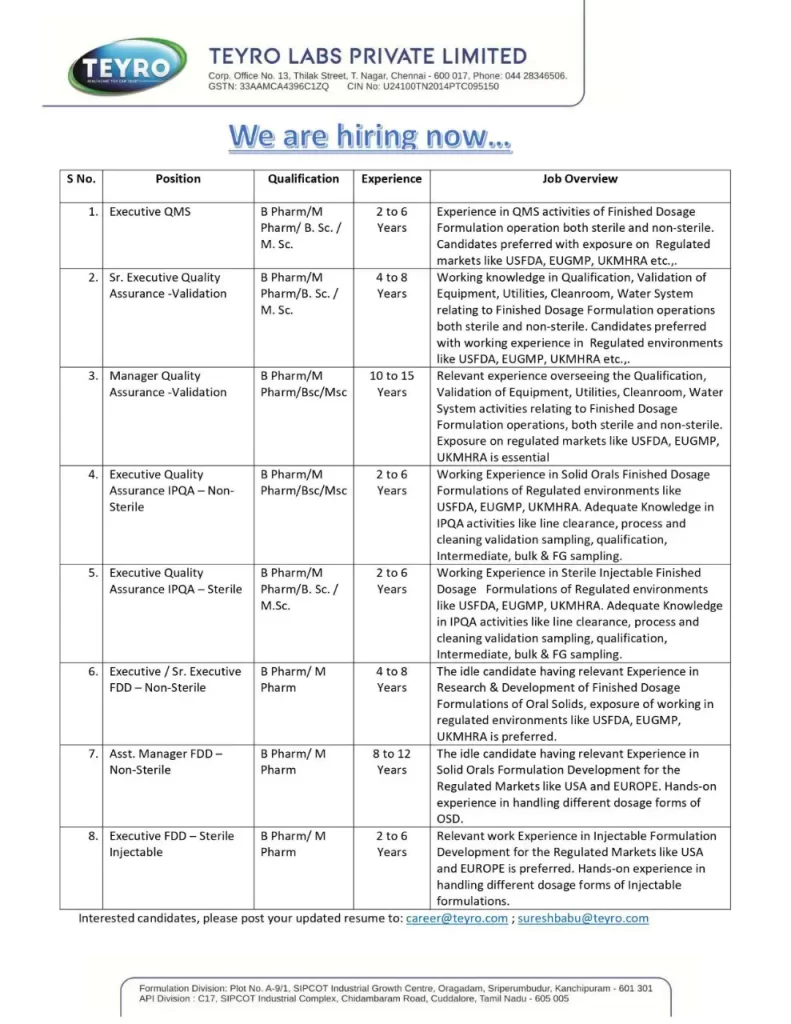
Executive QMS
Educational Qualification: B Pharmacy / M Pharm/ BSc / MSc
Experience: 2 to 6 Years
Job Description: Experience in QMS activities of Finished Dosage Formulation operation both sterile and non-sterile. Candidates preferred exposure to Regulated markets like USFDA, EUGMP, UKMHRA, etc…
Sr. Executive Quality Assurance -Validation
Education Qualification: B Pharmacy /M Pharm /BSc / MSc
Experience: 4 to 8 Years
Job Description: Working knowledge in Qualification, Validation of Equipment, Utilities, Cleanroom, and Water Systems relating to Finished Dosage Formulation operations both sterile and non-sterile. Candidates preferred with working experience in Regulated environments like USFDA, EUGMP, UKMHRA, etc.
Manager Quality Assurance -Validation
Educational Qualification: B Pharmacy / M Pharm / BSc / MSc
Experience: 10 to 15 Years
Job Description: Relevant experience overseeing the Qualification, Validation of Equipment, Utilities, Cleanroom, and Water System activities relating to Finished Dosage Formulation operations, both sterile and non-sterile. Exposure to regulated markets like USFDA, EUGMP, and UKMHRA is essential
Executive Quality Assurance IPQA-Non- Sterile
Required Education: B Pharmacy / M Pharm /BSc / MSc
Experience: 2 to 6 Years
Job Description: Working Experience in Solid Orals Finished Dosage Formulations of Regulated environments like USFDA, EUGMP, and UKMHRA. Adequate Knowledge in IPQA activities like line clearance, process, and cleaning validation sampling, qualification, Intermediate, bulk & FG sampling.
Executive Quality Assurance IPQA-Sterile
Educational Qualification: B Pharmacy /M Pharm / BSc / MSc
Experience: 2 to 6 Years
Job Description: Working Experience in Sterile Injectable Finished Dosage Formulations of Regulated environments like USFDA, EUGMP, and UKMHRA. Adequate Knowledge in IPQA activities like line clearance, process, and cleaning validation sampling, qualification, Intermediate, bulk & FG sampling.
Executive/Sr. Executive FDD-Non-Sterile
Education Qualification: B Pharm/ M Pharm
Experience: 04 to 08 years
Job Description: The idle candidate having relevant Experience in Research & Development of Finished Dosage Formulations of Oral Solids, and exposure to working in regulated environments like USFDA, EUGMP, and UKMHRA is preferred.
Asst. Manager FDD- Non-Sterile
Education: B Pharmacy / M Pharm
Experience: 8 to 12 Years
Job Description: The idle candidate has relevant Experience in Solid Orals Formulation Development for the Regulated Markets of USA and EUROPE. Hands-on experience in handling different dosage forms of OSD.
Executive FDD-Sterile Injectable
Education: B Pharm/M Pharm
Experience: 2 to 6 Years
Job Description: Relevant work Experience in Injectable Formulation Development for Regulated Markets like USA and EUROPE is preferred. Hands-on experience in handling different dosage forms of Injectable formulations.
application process; Interested candidates, please post your updated resume to: [email protected]; [email protected]
Plant Address: Formulation Division: Plot No. A-8/1, SIPCOT Industrial Growth Centre, Oragadam, Sriperumbudur, Kanchipuram-001 301 API Division: C17, SIPCOT Industrial Complex, Chidambaram Road, Cuddalore, Tamil Nadu – 605 005






