Hetero walk – QC,QA, Production & Engineering; Bangalore
Hetero walk in interview at Bangalore for QC, Production, Engineering & Quality Assurance Job Openings 2022
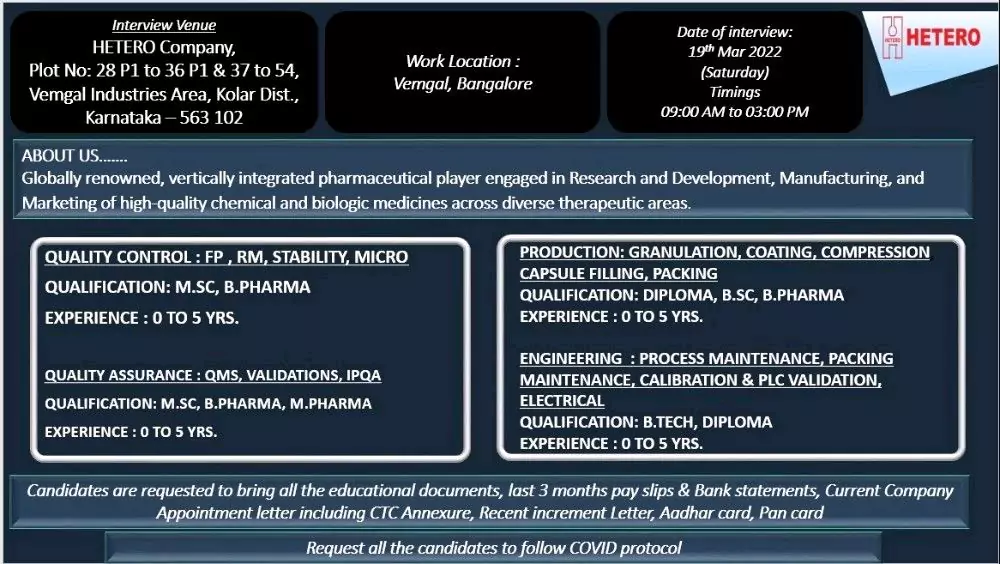
Globally renowned, vertically integrated pharmaceutical player engaged in Research and Development, Manufacturing, and Marketing of high-quality chemical and biologic medicines across diverse therapeutic areas.
VACANCY INFORMATION
QUALITY CONTROL: FP, RM, STABILITY, MICRO
QUALIFICATION: MSC, B Pharmacy
EXPERIENCE: 0 TO 5 YRS.
QUALITY ASSURANCE: QMS, VALIDATIONS, IPQA
QUALIFICATION: MSC, B PHARMA, M PHARMA
EXPERIENCE: 0 TO 5 YRS.
PRODUCTION: GRANULATION, COATING, COMPRESSION CAPSULE FILLING, PACKING
QUALIFICATION: DIPLOMA, BSC, B.PHARMA
EXPERIENCE: 0 TO 5 YRS.
ENGINEERING JOBS
ENGINEERING PROCESS MAINTENANCE, PACKING MAINTENANCE, CALIBRATION & PLC VALIDATION, ELECTRICAL
QUALIFICATION: B TECH, DIPLOMA
EXPERIENCE: 0 TO 5 YRS.
Candidates are requested to bring all the educational documents, last 3 months pay slips & Bank statements, Current Company Appointment letter including CTC Annexure, Recent increment Letter, Aadhar card, Pan card
Request all the candidates to follow COVID protocol
Work Location: Vemgal, Bangalore
Date of interview: 19th Mar 2022 (Saturday) Timings 09:00 AM to 03:00 PM
Interview Venue : HETERO Company, Plot No: 28 P1 to 36 P1 & 37 to 54, Vemgal Industries Area, Kolar Dist., Karnataka-563 102





