Enzene biosciences Walk-in drive in Hyderabad for Quality Control and Bio-Bulk Manufacturing
Enzene biosciences Walk-in drive in Hyderabad on May 6th and 7th for Quality Control and Bio-Bulk Manufacturing positions
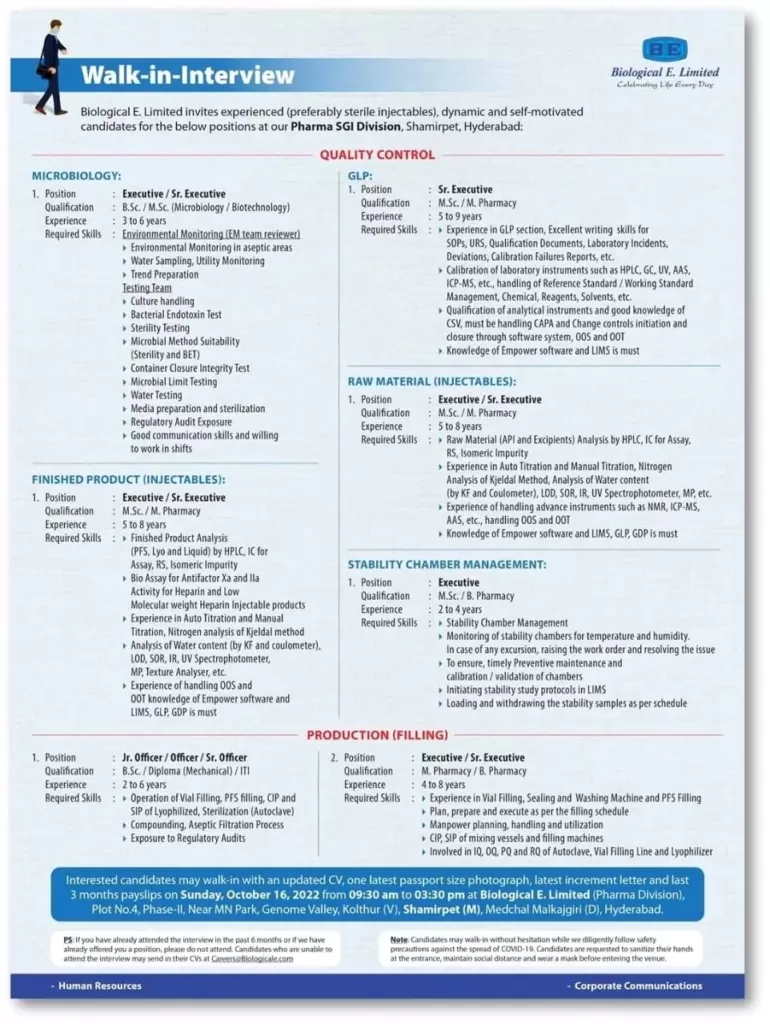
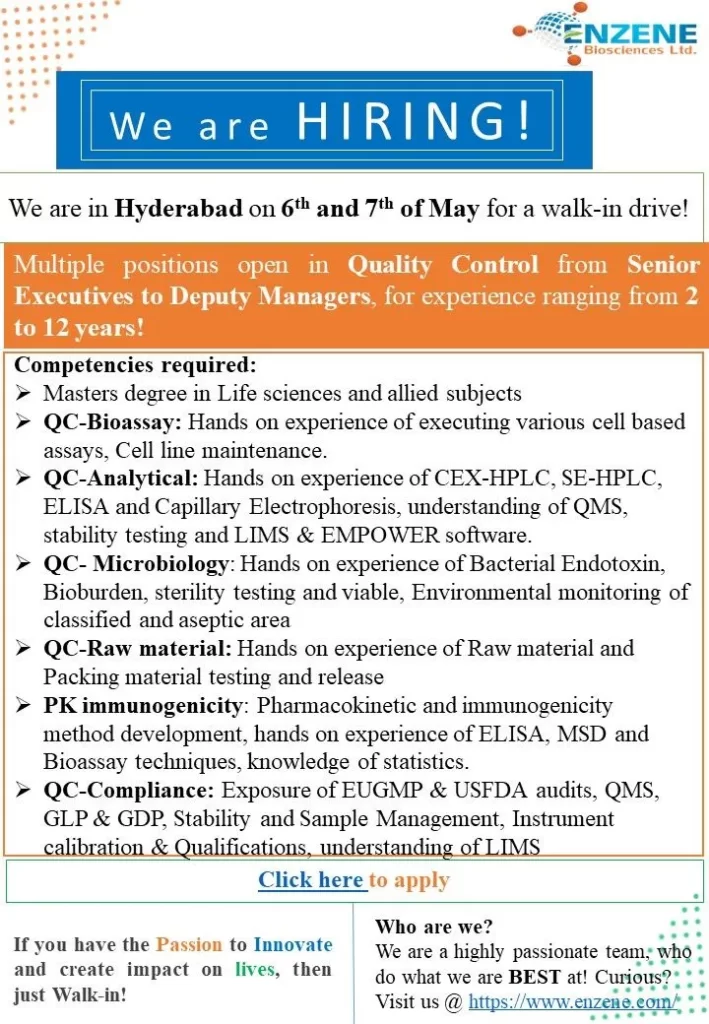
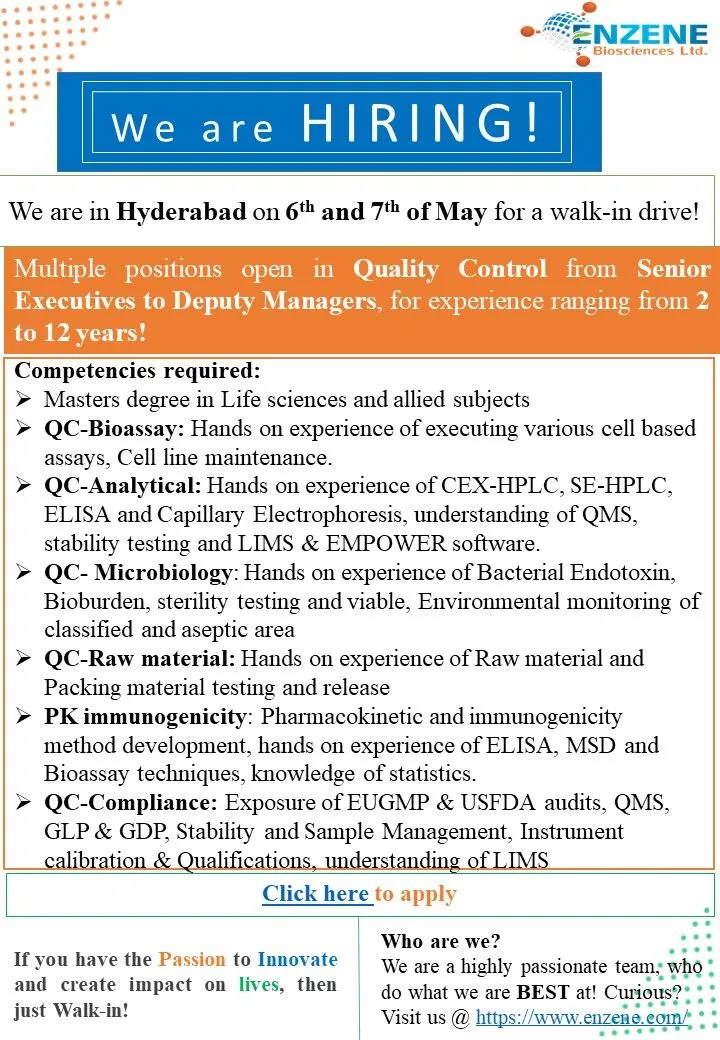
Quality Control Positions:
Senior Executive/Deputy Manager
Department: Quality Control – Bioassay
Experience: 4-10 years
Qualification: Master’s degree in Life sciences and allied subjects
Competencies required: Hands-on experience in executing various cell-based assays, cell line maintenance, CEX-HPLC, SE-HPLC, ELISA, Capillary Electrophoresis, understanding of QMS, stability testing, LIMS & EMPOWER software.
Senior Executive/Deputy Manager
Department: Quality Control – Analytical
Experience: 4-10 years
Qualification: Master’s degree in Life sciences and allied subjects
Competencies required: Hands-on experience of CEX-HPLC, SE-HPLC, ELISA, Capillary Electrophoresis, understanding of QMS, stability testing, LIMS & EMPOWER software.
Senior Executive/Deputy Manager
Department: Quality Control – Microbiology
Experience: 4-10 years
Qualification: Master’s degree in Life sciences and allied subjects
Competencies required: Hands-on experience of Bacterial Endotoxin, Bioburden, sterility testing, viable, Environmental monitoring of the classified and aseptic area.
Senior Executive/Deputy Manager
Department: Quality Control – Raw material
Experience: 4-10 years
Qualification: Master’s degree in Life sciences and allied subjects
Competencies required: Hands-on experience in Raw material and Packing material testing and release.
Senior Executive/Deputy Manager
Department: Quality Control – PK immunogenicity
Experience: 4-10 years
Qualification: Master’s degree in Life sciences and allied subjects
Competencies required: Pharmacokinetic and immunogenicity method development, hands-on experience of ELISA, MSD, and Bioassay techniques, knowledge of statistics.
Senior Executive/Deputy Manager
Department: Quality Control – Compliance
Experience: 4-10 years
Qualification: Master’s degree in Life sciences and allied subjects
Competencies required: Exposure to EUGMP & USFDA audits, QMS, GLP & GDP, Stability and Sample Management, Instrument
Bio-Bulk Manufacturing from Senior Executives to Deputy Managers
Multiple positions open in Bio-Bulk Manufacturing from Senior Executives to Deputy Managers, for experience ranging from 2 to 12 years!
Competencies required:
Master’s degree in Life sciences and allied subjects Experience in mammalian/microbial-based biopharmaceutical
production processes and working in a cGMP environment Operate process equipment like chromatography systems, filtration systems, autoclave, continuous centrifuge, etc.
Execute and manage media, buffer preparation, and filtration activities
Hands-on experience in large-scale chromatography column packing, and UF/DF unit operation.
Good documentation practices and preparation/review of documents like BMR, SOPs, and Protocols. Experience in equipment qualification and validations
Walk-in drive information;
Date; 6th and 7th of May